Bismuth
Bismuth:
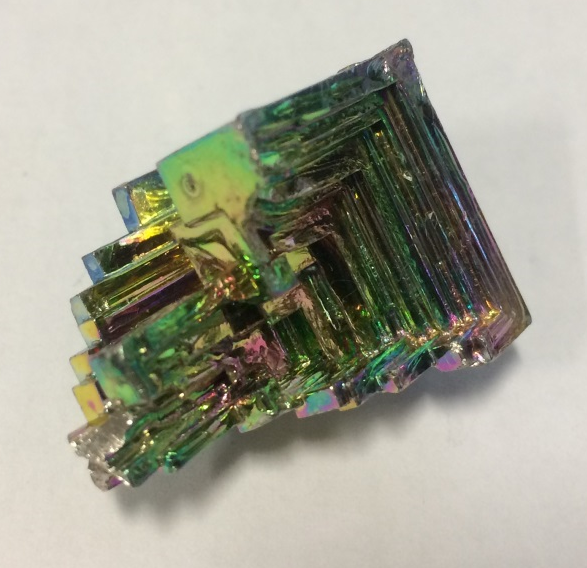
Image showing a lab-grown sample of bismuth, with iridescent sheen due to a surface layer of bismuth oxide.
Facts about Bismuth:
- Bismuth: metallic grey element
- Fun fact about Bismuth: Bismuth is used medically to treat some stomach upsets as bismuth Bismuth Subsalicylate (as Pepto bismol in the UK).
- Chemical symbol: Bi
- Atomic number: 83
A crystal structure containing Bismuth:

Crystal structure of bismuth oxychloride, BiOCl, showing bismuth, oxide and chloride ions in purple, red and green, respectively.
Facts about this structure:
- Formula: BiOCl
- Structure name: PbClF type
- Fun fact about the structure: Bismuth oxychloride BiOCl gives pearlescence in cosmetics, and currently investigated for its photocatalytic properties.
- ICSD number: 29143 (Find out more about the ICSD database)
- Associated publication: J. Y. Xiong, G. Cheng, G. F. Li, F. Quin, R. Chen, RSC Advances, 2011, 1, 1542, DOI: 10.1039/c1ra00335f
More about Bismuth:
Bismuth is one of the most strongly diamagnetic materials; it’s metallic but fairly brittle and a solid at standard conditions (but melts at 271°C). The metallic element is grey in colour but the iridescence on the surface of the sample shown above is due to a thin layer of bismuth oxide on the surface. Bismuth is said to be the heaviest element with a stable nucleus. Its most stable cation Bi3+ has an interesting electronic configuration which makes it useful in functional materials.
Learn More About the International Year of the Periodic Table (IYPT) in Crystals Project:
This project (#IYPTCrystals) is part of the International Year of the Periodic Table celebration (#IYPT2019), read more about the project here.
You can follow us on social media; search for #IYPTCrystals or follow The CCDC on X @ccdc_cambridge on Facebook ccdc.cambridge, on Instagram ccdc_cambridge or on YouTube CCDCCambridge.
Understand some of the terms and concepts used with our Frequently Asked Questions page here.
A 3D visualization showing Bismuth in real crystal structures: