Rutherfordium
Rutherfordium:

Ernest Rutherford, after whom the element Rutherfordium is named. (Photo courtesy: Library of Congress Prints and Photographs Division, Washington DC)
Facts about Rutherfordium:
- Rutherfordium: Rutherfordium is radioactive and unstable. Theoretical predictions expect it to be solid under ambient conditions, having chemical and physical properties similar to Hafnium.
- Fun fact about Rutherfordium: The name Rutherfordium was decided upon after a long debate between US and Soviet research groups – both of whom claimed to have discovered the element first. This controversy in the 1960s is sometimes known as the Transfermium Wars!
- Chemical symbol: Rf
- Atomic number: 104
A crystal structure celebrating Rutherfordium:
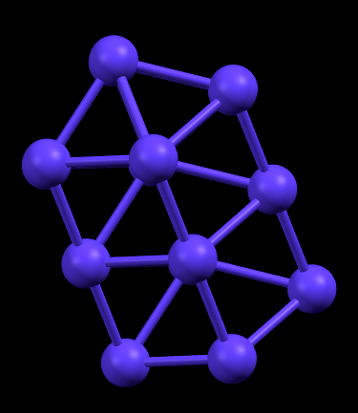
Hexagonal Close Packed (HCP) structure as observed in Cobalt. Rutherfordium is theoretically predicted to have the same crystal packing
Facts about this structure:
- Formula: Co
- Structure name: Cobalt
- Fun fact about the structure: The crystal structure of Rutherfordium is expected to be HCP due to its similarity with Hafnium which also forms HCP lattice. This similarity between members of the same group is a fascinating feature of the periodic table as we know it.
- ICSD number: 44990 (Find out more about the ICSD database)
- Associated publication: E.A. Owen, D. Madoc Jones, Proceedings of the Physical Society, London, 1954, 67, 459, Link to materials project: 10.17188/1263614
More about Rutherfordium:
The discovery of Rutherfordium in the 1960s was accompanied with a controversial naming debate between the US and Soviet groups who worked on the element. The final name was decided upon in 1997. Rutherfordium is radioactive with the most stable isotope (267Rf) having half life close to just an hour. Production of the element requires very expensive laboratory set-up, and this coupled with the inherent instability of Rutherfordium has left a principal part of its chemistry and physics unexplored. Few experiments indicate Rutherfordium having similar chemistry as other members of group IV in the periodic table. Theoretical calculations have indicated that the element may be more dense than the most dense element known till now (Osmium)! Since no Rutherfordium crystal structures have been determined yet, the 3D visualiser below contains another rather interesting Cobalt structure. This time it is a spectacular 36-nuclear complex of cobalt(II) which can be found in the Cambridge Structural Database (CSD) with Refcode LAXRAG and publication DOI:10.1016/j.poly.2017.03.056.
Learn More About the International Year of the Periodic Table (IYPT) in Crystals Project:
This project (#IYPTCrystals) is part of the International Year of the Periodic Table celebration (#IYPT2019), read more about the project here.
You can follow us on social media; search for #IYPTCrystals or follow The CCDC on X @ccdc_cambridge on Facebook ccdc.cambridge, on Instagram ccdc_cambridge or on YouTube CCDCCambridge.
Understand some of the terms and concepts used with our Frequently Asked Questions page here.
A 3D visualization showing Rutherfordium in real crystal structures: