Synthetic Routes to Pharmaceuticals Greatly Expanded Leading to New and More Effective Treatments
Crystallographers provide medicinal chemists with increase of pharmaceutical building blocks.
- New research published by the American Chemical Society (JACS Au) identifies a
hidden conglomerate pool of 1800 structures in the Cambridge Structural Database
(CSD). - These 1800 structures augment the limited biological chiral pool of synthetic
building blocks used by medicinal chemists in drug synthesis. - Medicinal chemists can now draw on this crystallographic knowledge to
synthesise drugs differently than before – more efficiently, in new
ways. - Opens new synthetic routes to existing drugs and could lead to new drugs for
more effective treatment of disease. - The collaborative research between the University of Durham and the CCDC has
recently been published (Identifying a Hidden Conglomerate Chiral Pool in the CSD – Mark P.
Walsh, James A. Barclay, Callum S. Begg, Jinyi Xuan, Natalie T. Johnson, Jason C. Cole,
and Matthew O. Kitching, JACS Au Article ASAP DOI:
10.1021/jacsau.2c00394).
Cambridge, UK. 28 September, 2022 – the
Cambridge Crystallographic Data Centre (CCDC) today highlights that a search of the
Cambridge Structural Database (CSD) has found nearly 1,800 conglomerate crystal
structures — molecules that have spontaneously enriched chirality upon
crystallization.
These 1,800 structures augment the limited biological chiral pool of synthetic
building blocks used by medicinal chemists in drug synthesis, opening new synthetic
routes to existing drugs and could lead to new drugs for more effective treatment of
disease.
This finding from a team led by Mark Walsh and Matthew Kitching from Durham
University, published in JACS Au, introduces a new and potentially unlimited pool of
chiral molecules outside of the chiral molecules derived from the natural world – the
biological chiral pool. This pool is used by medicinal chemists to introduce chirality
to their molecules.
Most biological substances are chiral, including protein binding sites, so
drugs that bind to these receptors must also be chiral. Famously the drug Thalidomide
was withdrawn from the market when one enantiomer was found to cause birth defects, only
20 years later did scientists find that the other chiral form is safe.
One way used by medicinal chemists to obtain enriched chiral molecules during
synthesis is by conglomerate crystallization (where molecules spontaneously crystallise
into single enriched crystals) or by using chiral compounds from the limited chiral
pool. This research adds a significant alternative source of chirality.
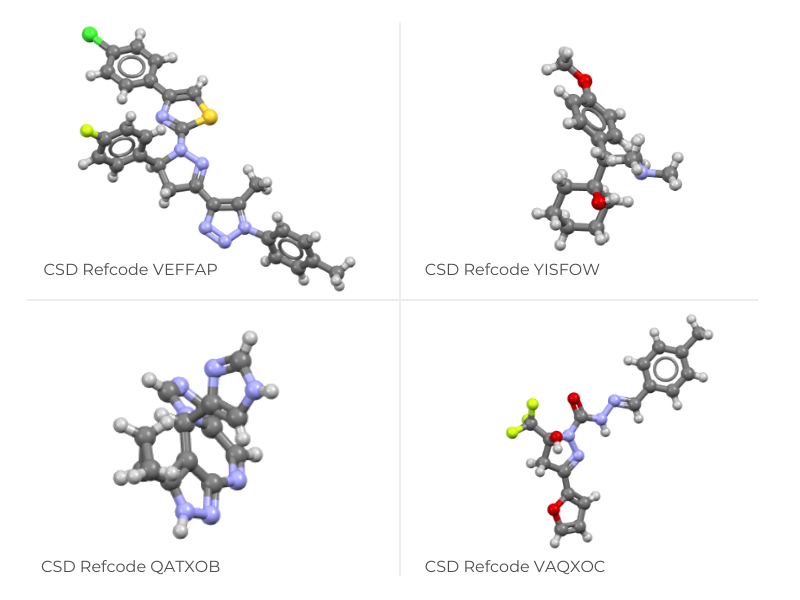
medically relevant crystal structures in the CSD that were identified as conglomerates.
These structures have spontaneously enriched chirality on crystallization, presenting
more starting materials that medicinal chemists could use in drug
design.
The researchers first mined the ~1.2 million crystal structures in the CSD for
those in Sohncke space groups with the potential to be chiral. This produced a subset of
over 21,000 crystal candidates that then had their synthesis examined (by reference to
the primary literature) to identify those that were produced by conglomerate
crystallisation. 1800 compounds were identified that were synthesised by racemic methods
but spontaneously crystallised in an enriched chiral form.
“We hope that the curation of this list of conglomerate crystals aids
the development of preferential crystallisation and spontaneous deracemization
protocols, while also furthering the understanding of the formation of conglomerate
crystal behaviour,”Mark Walsh, Durham University
“I’m delighted to see that the value of the 1.2 million crystal
structures in the CSD is once again being utilised in another scientific field beyond
crystallography. Medicinal chemists can now use the CSD to greatly widen their options
to introduce chirality to their molecules than previously,”Dr Jürgen Harter, CEO, CCDC.
Read the open access paper in JACS Au here.
- Interviews with CCDC scientists and C-level executives available upon
request. - Stunning molecular images for both print and electronic use available upon
request (credited: Image generated using CCDC’s Mercury. Courtesy of the The Cambridge Crystallographic Data Centre
(CCDC)). - Contact hello@ccdc.cam.ac.uk with any
enquiries.