FDA Novel Drug Approvals 2024 — Small Molecules Rise to 64%
The Food and Drug Administration (FDA) in the US approved 50 new drugs throughout 2024 [1]. This figure, despite being lower than the 55 approvals for 2023, brings the ten-year rolling average for new approvals up to 46.5 per year, the highest in over 20 years [2].
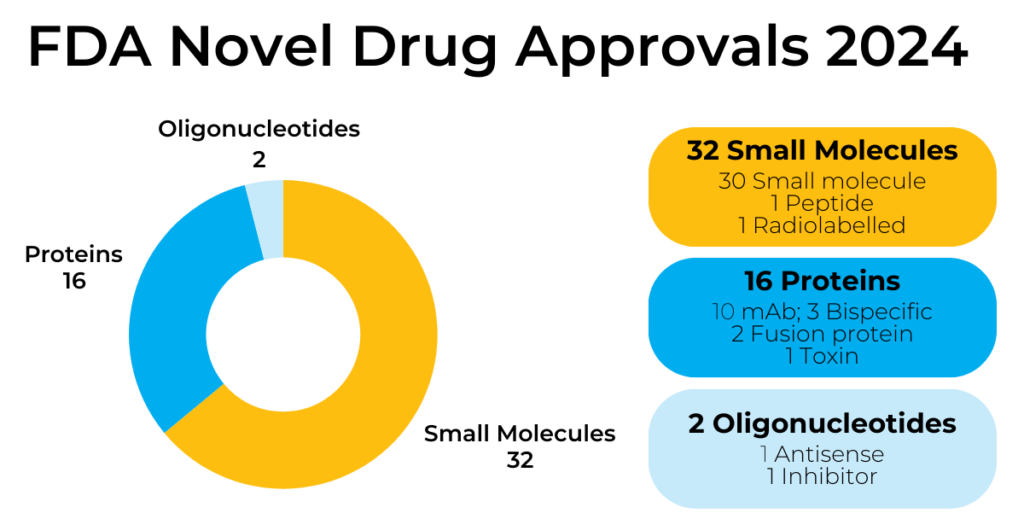
As reported in Nature Reviews Drug Discovery, the list of new drugs that can be seen in Figure 1 comprises 32 small molecules (64% of the total, which includes peptides of up to 40 amino acids in length), 16 proteins (32%), and 2 oligonucleotides (4%) [2].
Oncology remains the therapeutic area with the highest number of approvals (15, corresponding to 30% of the total), followed by dermatology and haematology (each counting for 6 approvals, 12%), and cardiovascular (5 approvals, 10%) [2].
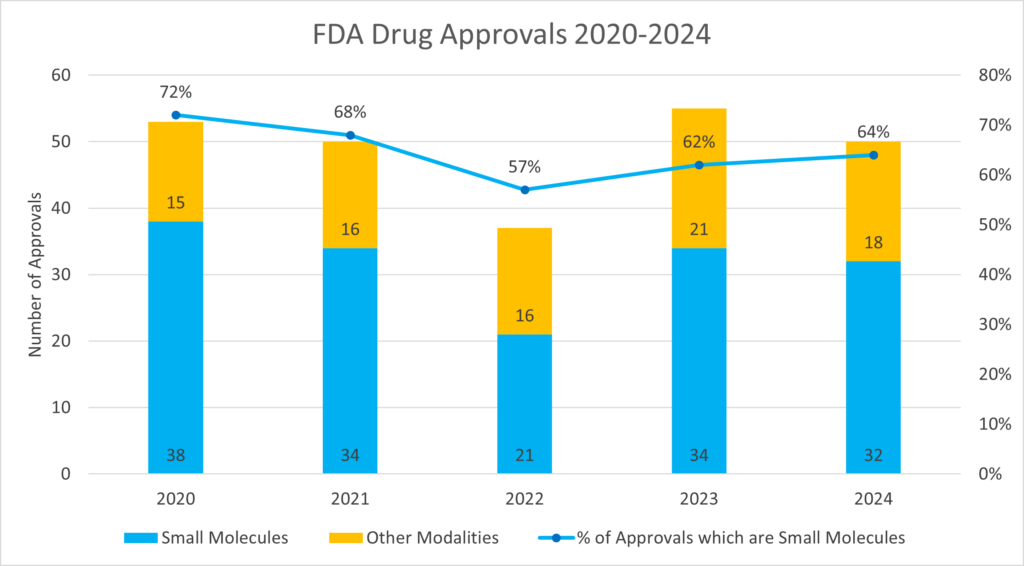
From the data reported in Figure 2, it can be seen that since 2022 both the number of approved small molecules and the total approvals have increased, and then decreased. Nevertheless, the percentage of small molecules has progressively increased, going form 57% in 2022, to 62% in 2023, and to 64% in 2024.
Beside this rising trend, the data averaged over the past 5 years show that 64.6% of the FDA approvals are small molecules, demonstrating the high impact that these continue to have in pharmaceutical discoveries.
Availability in the Cambridge Structural Database
The Cambridge Structural Database (CSD) contains 7 of the 32 small molecule drugs approved by the FDA at the time of writing.
The CSD is the world’s largest database of small molecule organic and metal-organic crystal structures. Each is from real-world experimental determinations in the literature or directly deposited in the database, and every entry is curated by our team to ensure accuracy and adherence to FAIR data principles. The learnings from the data as a whole are used in novel drug discovery and many other applications.
The structures of the small molecule drugs approved by the FDA that are in the CSD can be viewed using the CSD refcodes listed below.
Drug (brand name) – Resmetirom (Rezdiffra)
Rezdiffra is a thyroid hormone receptor-beta (THR-beta) agonist used to treat noncirrhotic non-alcoholic steatohepatitis with moderate to advanced liver scarring [1].
CSD refcodes: POHPEL, POHNEJ (ethanol solvate), POHNIN (ethyl acetate solvate), POHNOT (dimethylacetamide solvate), POHNUZ (dioxane solvate), POHPAH (tetrahydrofuran solvate), POHPIP (methyl isopropyl ketone solvate).
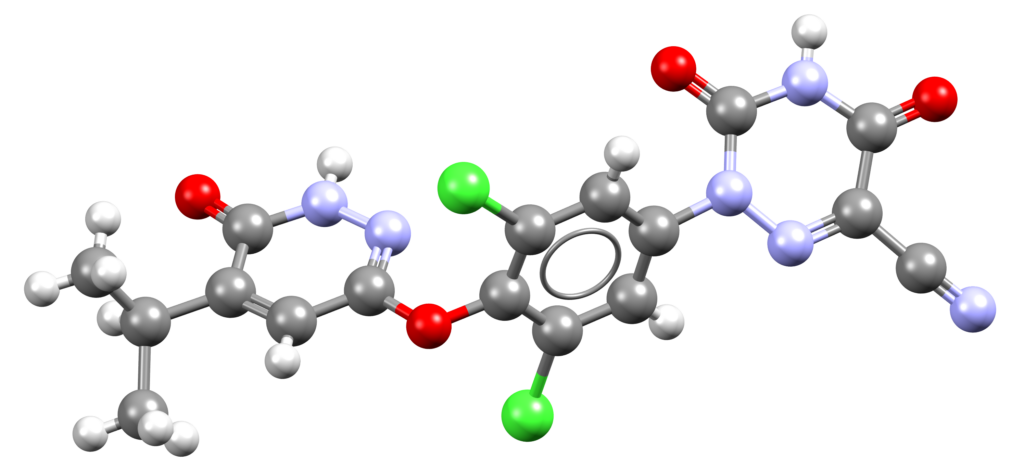
Ensifentrine (Ohtuvayre)
Ohtuvayre is a phosphodiesterase 3 (PDE3) inhibitor and phosphodiesterase 4 (PDE4) inhibitor used to treat chronic obstructive pulmonary disease in adult patients [1].
CSD entry: GOXTUM (chloride salt, methanol solvate, hydrate).
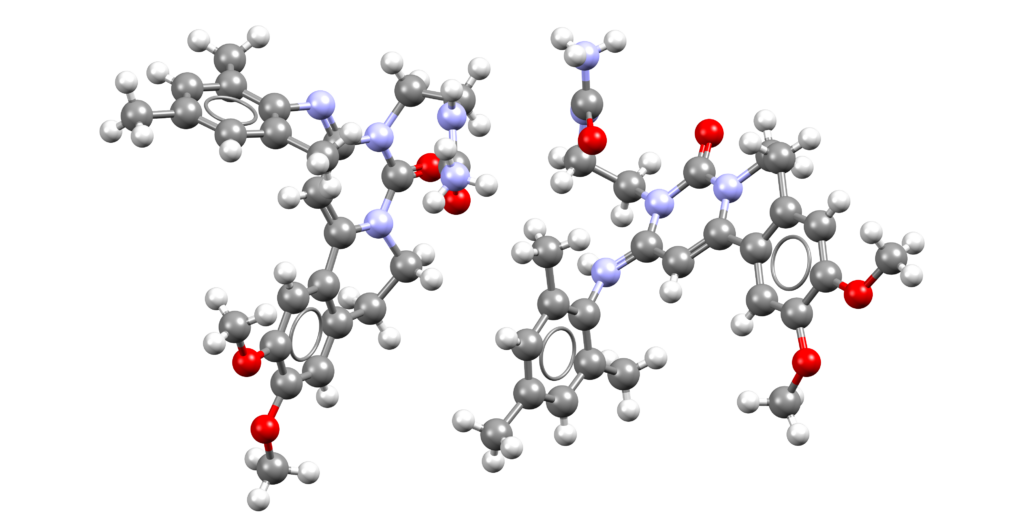
Levacetylleucine (Aqneursa)
Aqneursa is indicated for the treatment of neurological manifestations of Niemann-Pick disease type C (NPC) [1].
CSD entries: WOMXEC (salt), RARZET (salt).
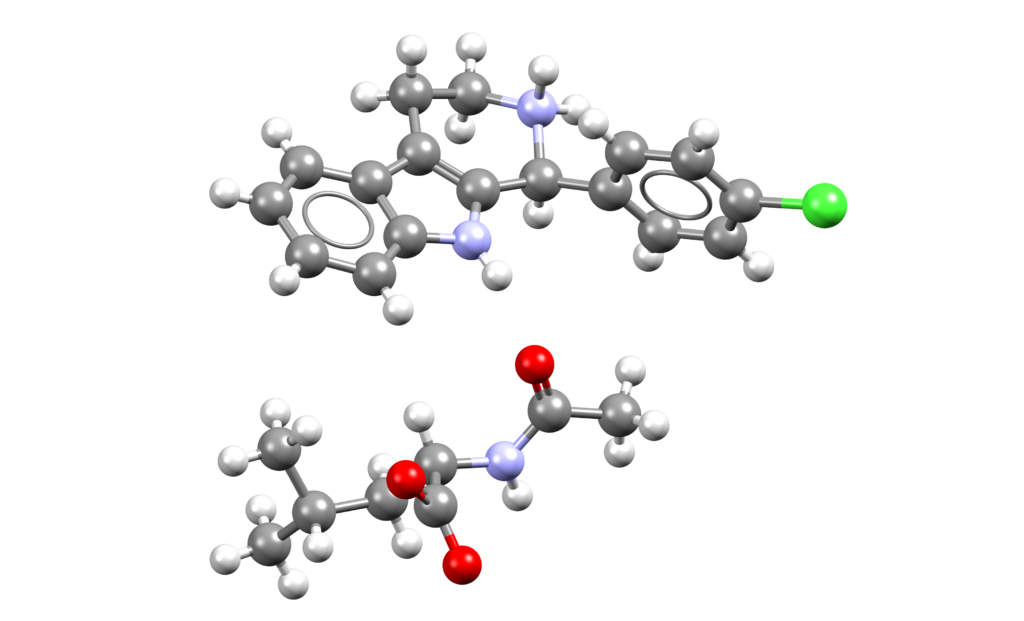
Xanomeline and trospium chloride (Cobenfy)
Cobenfy is a combination of xanomeline, a muscarinic agonist, and trospium chloride, a muscarinic antagonist, indicated for the treatment of schizophrenia in adults [1].
CSD entries for trospium: EZOLIQ (chloride salt, 4-aminobenzoic acid co-crystal, hydrate), EZOLOW (chloride salt, benzoic acid co-crystal), EZOLUC (chloride salt, acetone solvate), EZOMAJ (saccharinate hydrate), EZOMAJ01 (saccharinate hydrate), IPIKOJ (chloride salt, acetonitrile solvate), IPIKUP (chloride salt, isopropanol solvate), IPILAW (chloride salt, methanol solvate), IPILEA (chloride salt, nitromethane solvate), IPILIE (chloride salt, propionitrile salt), IPILOK (chloride salt, hydrate), IPILUQ (chloride salt, hydrate), KIXYUN (chloride salt, ethanol solvate), XOGLOW (chloride salt, glutaric acid co-crystal), XOGLUC (chloride salt, adipic acid co-crystal), XOHQAO (chloride salt, salicylic acid co-crystal), XOHQES (chloride salt, oxalic acid co-crystal).
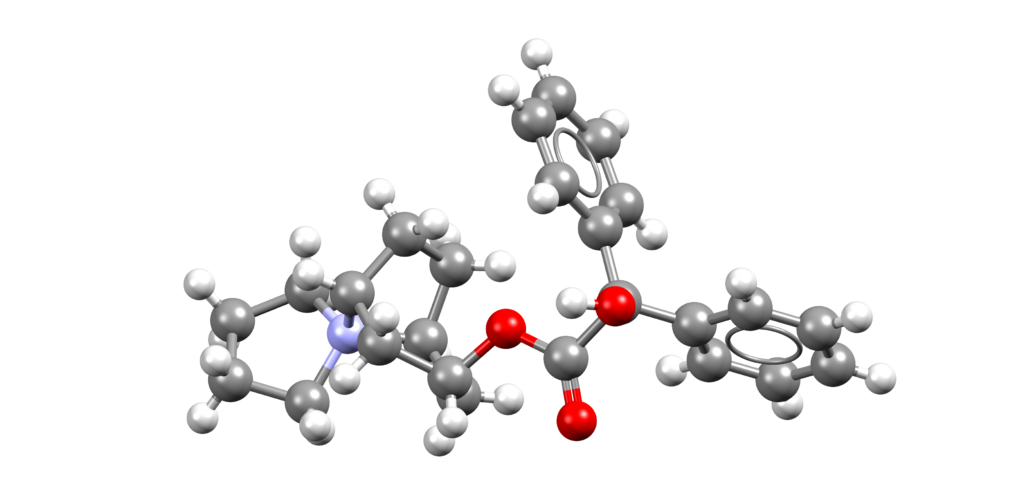
Sulopenem etzadroxil and probenecid (Orlynvah)
Orlynvah is a combination of sulopenem etzadroxil, a penem antibacterial, and probenecid, a renal tubular transport inhibitor, indicated for the treatment of uncomplicated urinary tract infections [1].
CSD entries for probenecid: QQQBSS, QQQBSS01, QQQBSS02, QQQBSS03, QQQBSS04, HEMQIC (N,N-dimethylformamide solvate), JIGKES, JIGKES01, JIGKES02, JIGKES03, JIGKES04 (co-crystals), KANBEJ (salt), KANBIN, KANBIN01 (4,4′-bipyridine co-crystals), KANBOT (acridine co-crystal), KANBUZ (pyridine solvate), QOWMEY (salt), QOXBIS (salt).
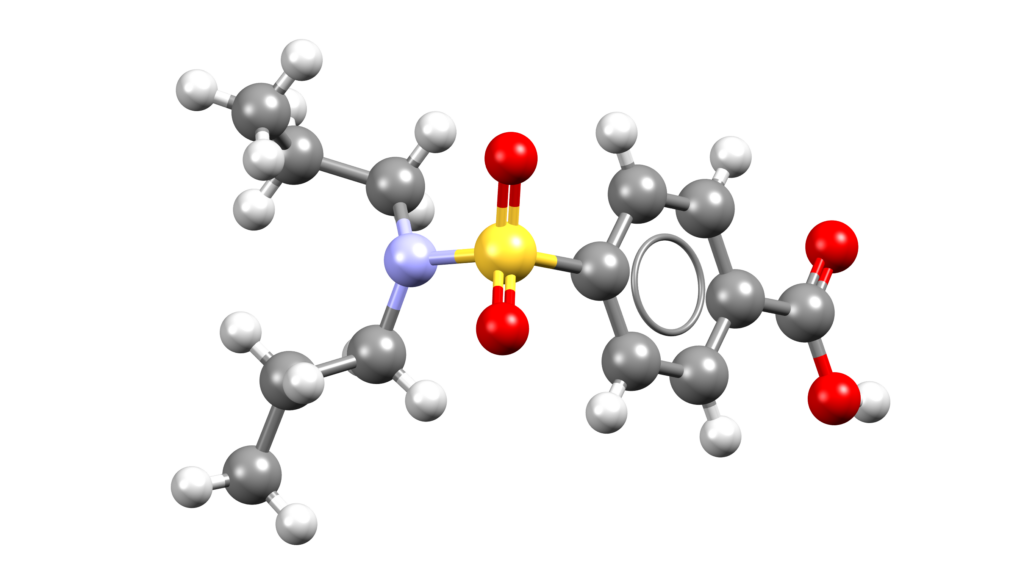
Iomeprol (Iomervu)
Iomervu is a radiographic contrast agent indicated for intra-arterial procedures and intravenous procedures [1].
CSD entries: EYUPUL, EYUPUL02.
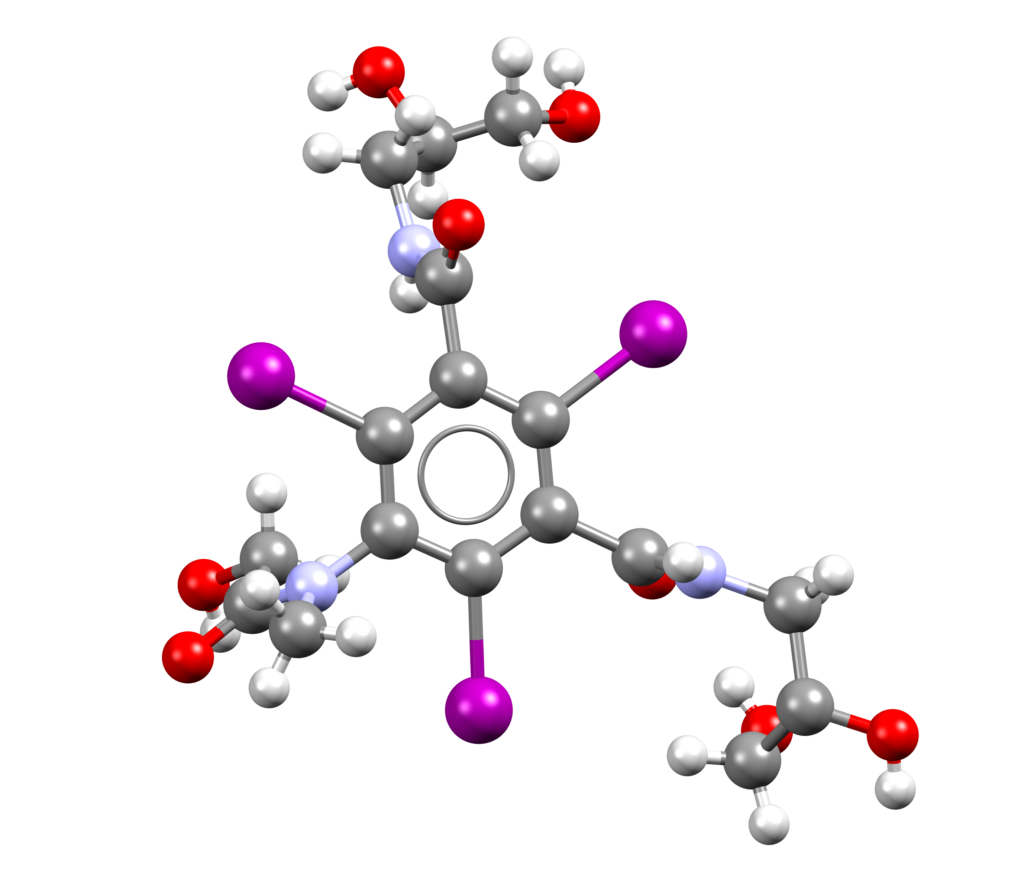
Deutivacaftor, vanzacaftor, and tezacaftor (Alyftrek)
Alyftrek is a combination of deutivacaftor, tezacaftor, and vanzacaftor indicated for the treatment of cystic fibrosis [1].
CSD entry for tezacaftor: GEMMAQ (no coordinates available).
Next Steps
Learn more about the CSD and its applications.
Subscribe to our newsletter to stay up to date on the latest news, events, webinars, and CSD data and software updates.
To discuss further and/or request a demo with one of our scientists, please contact us via this form or .
References
[1] https://www.fda.gov/drugs/novel-drug-approvals-fda/novel-drug-approvals-2024.