Aluminum
Aluminum:

The Command and Service Module from the Apollo 11 mission. The Command Module was made of an aluminum honeycomb sandwich bonded between sheet aluminum alloy.
Facts about Aluminum:
- Aluminum: Silvery-white metal
- Fun fact about Aluminum: Without electricity aluminum is difficult to extract from it’s ores (such as bauxite) which meant for over 20 years after it was first isolated in 1827 it was more expensive than gold!
- Chemical symbol: Al
- Atomic number: 13
A crystal structure containing Aluminum:
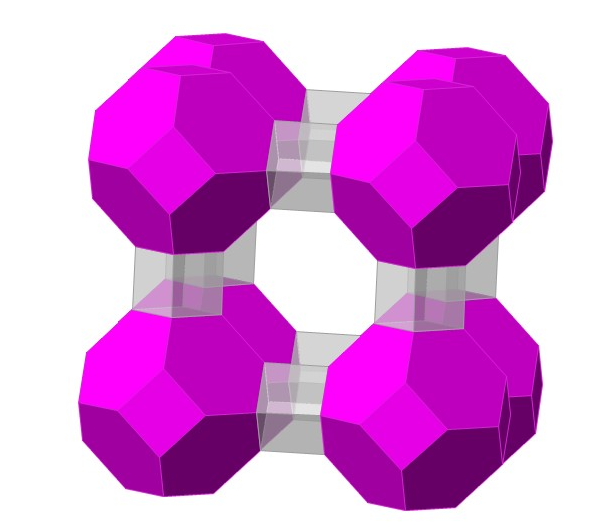
The massive polyhedra structure of zeolite A
Facts about this structure:
- Formula: [Na+12(H2O)27]8 [Al12Si12O48]8
- Structure name: Linde Type A (zeolite A)
- Fun fact about the structure: Chances are you have some zeolite A at home, where it acts as a water softener in washing detergent. Zeolites are naturally occurring water containing frameworks made out of aluminum, silicon and oxygen. They have a structure filled with holes and channels, a bit like a sponge, good at storing, sorting and swapping different chemicals. Zeolite A was one of the first synthesised without any natural forms known.
- ICSD number: 26911 (Find out more about the ICSD database)
- Associated publication: T. B. Reed, D. W. Breck, J. Am. Chem. Soc. (1956), 78, 5972-5977, DOI: 10.1021/ja01604a002
More about Aluminum:
Aluminum is very much the material of the 21st century, we use this silvery-white metal in all kinds of things, from drinks cans to spacecraft. Aluminum’s wide use is all down to it’s low density and it’s ability to resist corrosion due to a thin, protective layer of aluminum oxide that it forms – like a protective shield! Aluminum is one of the three main elements present in a class of minerals called zeolites. These zeolites are nets of aluminum and silicon, linked by oxygen which act like sponges, trapping other chemicals within them or sorting them like a sieve. They are a bit of a secret hero material, helping us in the everyday by producing petrol, softening water and even clotting blood in dressings or cleaning up radioactive waste!
Learn More About the International Year of the Periodic Table (IYPT) in Crystals Project:
This project (#IYPTCrystals) is part of the International Year of the Periodic Table celebration (#IYPT2019), read more about the project here.
You can follow us on social media; search for #IYPTCrystals or follow The CCDC on X @ccdc_cambridge on Facebook ccdc.cambridge, on Instagram ccdc_cambridge or on YouTube CCDCCambridge.
Understand some of the terms and concepts used with our Frequently Asked Questions page here.
A 3D visualization showing Aluminum in real crystal structures: