Argon
Argon:

Argon glows purple when placed in an electrical field.
Facts about Argon:
- Argon: Colourless, odorless, nonflammable and nontoxic gas
- Fun fact about Argon: Argon was named after the Greek word ‘argos’, meaning ‘lazy’, because it did nothing when it was combined with other elements.
- Chemical symbol: Ar
- Atomic number: 18
A crystal structure containing Argon:
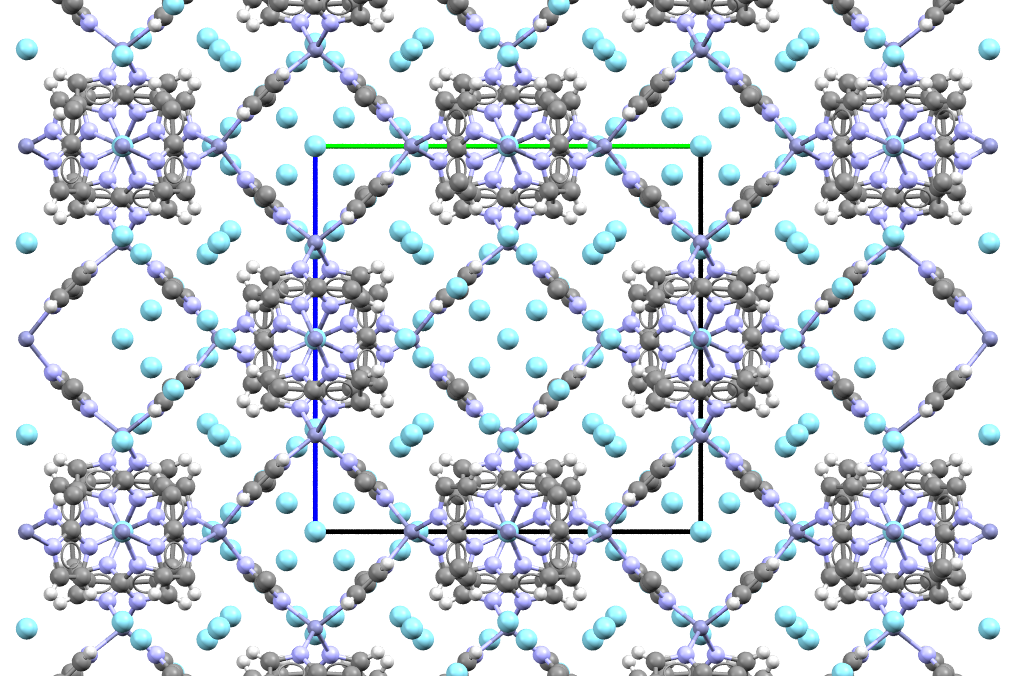
The material (grey, light-blue and white spheres) traps argon (cyan-blue spheres) like a sponge traps water!
Facts about this structure:
- Formula: C48H60N24Zn6.33Ar
- Structure name:catena-(bis(mu-2-methylimidazolato)-zinc argon)
- Fun fact about the structure: The open spaces in this structure are big enough to accommodate argon atoms in many different positions, which is why it looks so packed!
- CSD refcode: NIFPEA (What’s this?)
- Associated publication: Claire L. Hobday, Christopher H. Woodall, Matthew J. Lennox, Mungo Frost, Konstantin Kamenev, Tina Düren, Carole A. Morrison, Stephen A. Moggach, Nature Communications, 2018, 9, 1429, DOI: 10.1038/s41467-018-03878-6
More about Argon:
Argon, like the rest of the noble gases in column 18 in the periodic table, rarely interacts with other elements. To make this crystal, scientists put a sponge-like material between two diamonds and squeezed the diamonds very tightly together. This causes pressure to build up in the crystal. However, the argon needs to be cooled to-196.15 °C/ -321.07 °F, when it becomes a liquid, before it is possible to trap it inside the crystal. The fact that such harsh conditions are necessary to get argon into a crystal tell us that argon really doesn’t like to play with other elements! For this reason, it is often used as a carrier gas, i.e. it transports gases which are very reactive, as we know that argon will not interfere in chemical reactions.
Learn More About the International Year of the Periodic Table (IYPT) in Crystals Project:
This project (#IYPTCrystals) is part of the International Year of the Periodic Table celebration (#IYPT2019), read more about the project here.
You can follow us on social media; search for #IYPTCrystals or follow The CCDC on X @ccdc_cambridge on Facebook ccdc.cambridge, on Instagram ccdc_cambridge or on YouTube CCDCCambridge.
Understand some of the terms and concepts used with our Frequently Asked Questions page here.
A 3D visualization showing Argon in real crystal structures: