Astatine
Astatine:

Astatine is radioactive and has a half life of 8.1 hours
Facts about Astatine:
- Astatine: Radioactive, unstable
- Fun fact about Astatine: Less than 1g is predicted to be in the Earth’s crust at any one time
- Chemical symbol: At
- Atomic number: 85
A crystal structure celebrating Astatine:
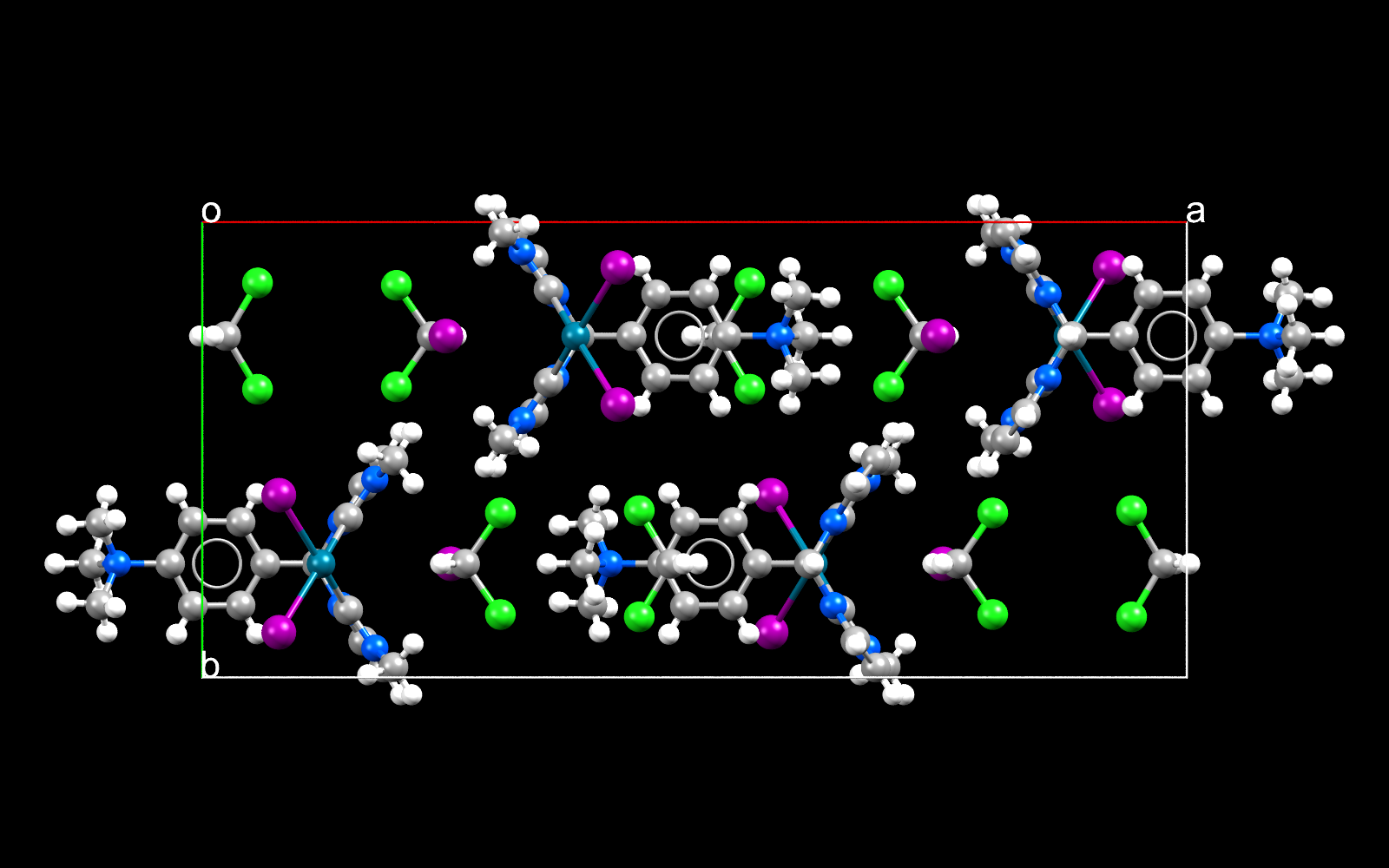
Palladium iodides are important in organic chemistry for example coupling 2 molecules together.
Facts about this structure:
- Formula: C18 H24 I2 N5 Pd +,I –,2(C H2 Cl2)
- Structure name: (1,1′-((4-(trimethylazaniumyl)phenyl)methylene)bis(3-methyl-2,3-dihydro-1H-imidazol-2-ylidene))-di-iodo-palladium iodide dichloromethane solvate
- Fun fact about the structure: The heavy metal palladium allows carbon to have less than its usual 4 covalent bonds
- CSD refcode: BUPHEB (What’s this?)
- Associated publication: Sai Puneet Desai, Moumita Mondal, Joyanta Choudhury, Organometallics, 2015, 34, 2731, DOI: 10.1021/om501163m
More about Astatine:
Astatine is the least reactive of the halogen elements and it is assumed to behave similarly to iodine. As it has such a short half-life no crystal structures of its compounds exist and very few compounds have been identified at all. Compounds of astatine with palladium are known and their relatives – palladium iodides – are incredible versatile reagents for organic synthesis. For instance this compound was developed to help modify aromatic compounds.
Learn More About the International Year of the Periodic Table (IYPT) in Crystals Project:
This project (#IYPTCrystals) is part of the International Year of the Periodic Table celebration (#IYPT2019), read more about the project here.
You can follow us on social media; search for #IYPTCrystals or follow The CCDC on X @ccdc_cambridge on Facebook ccdc.cambridge, on Instagram ccdc_cambridge or on YouTube CCDCCambridge.
Understand some of the terms and concepts used with our Frequently Asked Questions page here.
A 3D visualization showing Astatine in real crystal structures: