Barium
Barium:

Han purple is a pigment made from barium copper silicate. It was used by the imperial Chinese on high status items such as the uniforms of the Terracotta Warriors.
Facts about Barium:
- Barium: A soft silver-white metal with a golden sheen when pure (but dark grey when exposed to air).
- Fun fact about Barium: Glows in the dark. A form of the barium mineral baryte, ‘Bologna stones’ luminesces after exposure to sunlight and mystified 17th Century Italian alchemists.
- Chemical symbol: Ba
- Atomic number: 56
A crystal structure containing Barium:
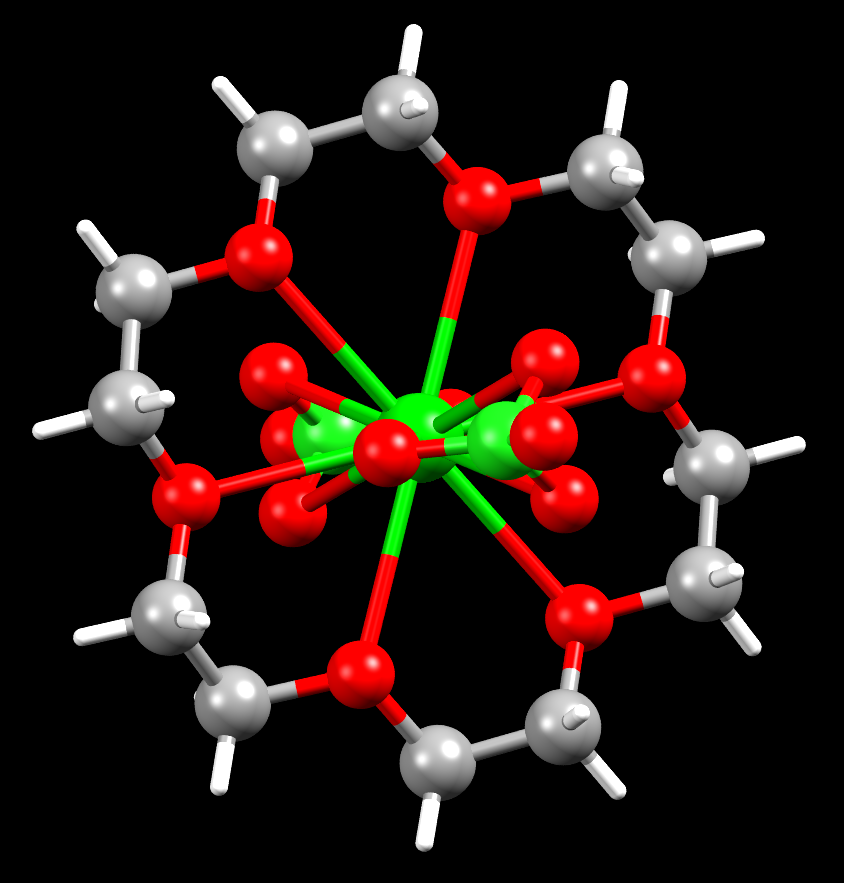
A barium crown ether complex
Facts about this structure:
- Formula: C12 H24 Ba Cl2 O14
- Structure name: (18-Crown-6)-bis(perchlorato-O,O’,O”)-barium
- Fun fact about the structure: Barium’s affinity for oxygen is shown in this structure, where the barium atom bonds to 12 oxygen atoms!
- CSD refcode: MIFNUL01 (What’s this?)
- Associated publication: P.C.Junk, J.W.Steed, Journal of Coordination Chemistry, 2007, 60, 1017, DOI: 10.1080/00958970600990226
More about Barium:
Barium is a metal with good electric conductance, like copper or gold, but it is much softer than those elements. Its Mohs hardness is 1.25, which means you can scatch it with your fingernail. It is also way more reactive than gold: if you put pure silvery-golden coloured barium in air, it immediately tarnishes to almost black as the barium reacts with oxygen. This reactivity has been used in the past in the manufacture of vacuum tubes for pre-transistor electronics; barium is a ‘getter’ that can suck up gases to improve the vacuum in the tube.
The best known use of barium is in X-ray imaging. Patients are given a ‘barium meal’ of barium sulphate so that medics can see details of the gut. It has to be barium sulphate, because soluble barium compounds are quite poisonous, as barium ions will block potassium sites in the nervous system leading to tremors, heart irregularities and even paralysis.
Learn More About the International Year of the Periodic Table (IYPT) in Crystals Project:
This project (#IYPTCrystals) is part of the International Year of the Periodic Table celebration (#IYPT2019), read more about the project here.
You can follow us on social media; search for #IYPTCrystals or follow The CCDC on X @ccdc_cambridge on Facebook ccdc.cambridge, on Instagram ccdc_cambridge or on YouTube CCDCCambridge.
Understand some of the terms and concepts used with our Frequently Asked Questions page here.
A 3D visualization showing Barium in real crystal structures: