Beryllium
Beryllium:

A gorgeous green emerald at the front of a diamond studded ring, beryllium is a key component of both emeralds and aquamarine.
Facts about Beryllium:
- Beryllium: White-grey metal at standard conditions
- Fun fact about Beryllium: Rare, beautiful and very toxic if mishandled, beryllium is an element you don’t often find in the everyday. It is incredibly light and strong and because it doesn’t absorb X-rays strongly is found as a ‘window’ in almost every X-ray instrument.
- Chemical symbol: Be
- Atomic number: 4
A crystal structure containing Beryllium:
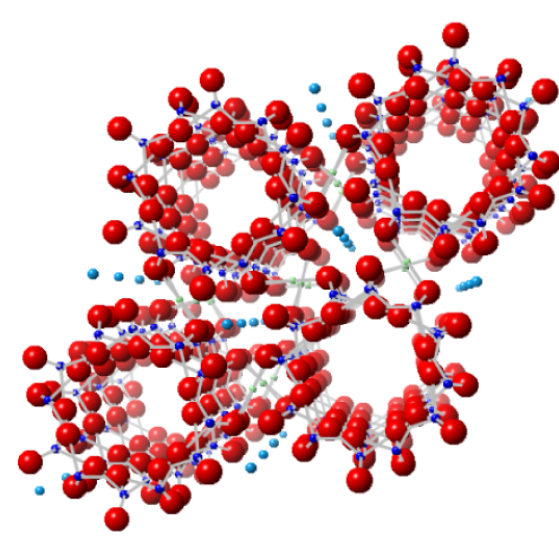
Image showing the first atomic structure of beryl, published by William Bragg in 1926
Facts about this structure:
- Formula: Al2 Be3 O18 Si6
- Structure name: Beryl
- Fun fact about the structure: The first X-ray derived atomic structure of the mineral beryl (emerald) was published by William Bragg in 1926. W. Bragg and his son Lawrence Bragg are the only father-son team to have jointly won a Nobel prize!
- ICSD number: 31891 (Find out more about the ICSD database)
- Associated publication: W.L. Bragg, Proceedings of the Royal Society London,Series A, 1926, 111, 691, DOI: 10.1098/rspa.1926.0088
More about Beryllium:
Beryllium is one of the most incredible elements you may never have heard of. Originally called glaucinium as its compounds tasted sweet (think glucose), this strong and lightweight element has found use in spacecraft and nuclear reactors as well as being a component of some of the most popular gemstones. Beryllium can be highly toxic in certain forms and so isn’t found in many everyday items, but it is an essential component of satellite-based telescope mirrors which makes the exploration of our universe possible. Emeralds, one of the most famous gemstones containing beryllium are actually rarer than diamonds!
Learn More About the International Year of the Periodic Table (IYPT) in Crystals Project:
This project (#IYPTCrystals) is part of the International Year of the Periodic Table celebration (#IYPT2019), read more about the project here.
You can follow us on social media; search for #IYPTCrystals or follow The CCDC on X @ccdc_cambridge on Facebook ccdc.cambridge, on Instagram ccdc_cambridge or on YouTube CCDCCambridge.
Understand some of the terms and concepts used with our Frequently Asked Questions page here.
A 3D visualization showing Beryllium in real crystal structures: