Boron
Boron:

Elemental crystalline Boron
Facts about Boron:
- Boron: Metalloid, with a high melting point and an empty P-orbital
- Fun fact about Boron: One of the key elements in Borax which is used to make slime.
- Chemical symbol: B
- Atomic number: 5
A crystal structure containing Boron:
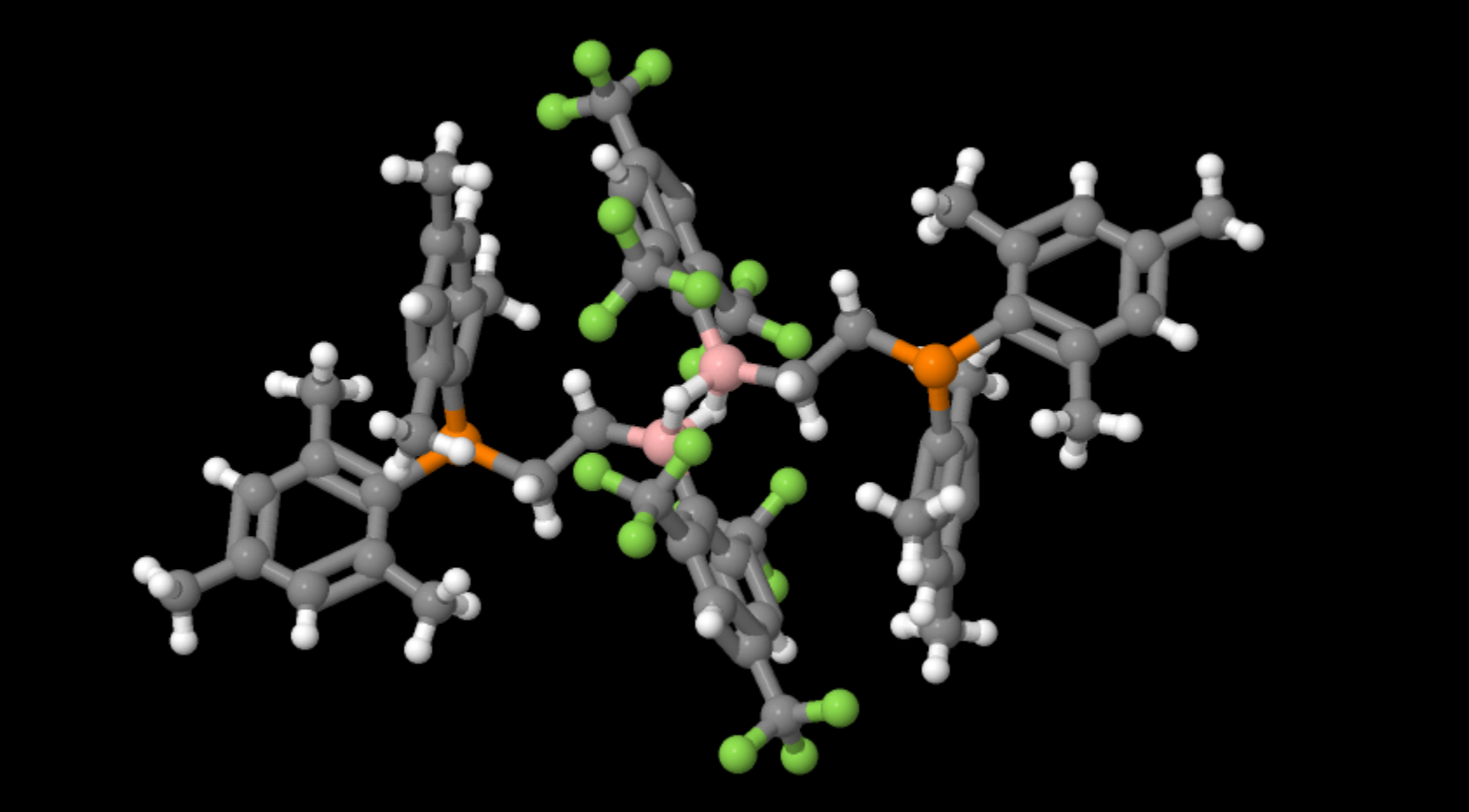
A diborane illustrating the ‘Banana bonding’ of 2 borons bridged by 2 hydrogens
Facts about this structure:
- Formula: C58 H58 B2 F18 P2
- Structure name: bis(μ-hydrido)-bis(2,4,6-tris(trifluoromethyl)phenyl)-bis(2-[bis(2,4,6-trimethylphenyl)phosphanyl]ethyl)-di-boron
- Fun fact about the structure: This compound is a mild reductant capable of making fomaldehyde from carbon monoxide
- CSD refcode: DOBDOQ (What’s this?)
- Associated publication: Gerhard Erker, Jun Li, Constantin G. Daniliuc, Gerald Kehr, Angewandte Chemie, International Edition, 2019, 58, 6737, DOI: 10.1002/anie.201901634
More about Boron:
Boron’s chemistry is largely governed by it only having 3 electrons in its outer shell. As a result it tends to form 3 covalent bonds leaving one p-orbital unoccupied which can accept lone pairs of electrons. What is unusual in diboranes is that whilst a covalently bound hydrogen has no lone pairs, two molecules containing boron hydrogen bonds can share hydrogens in a form known colloquially as banana bonding due to its shape. This was first proposed by Longuet-Higgins while still an undergraduate chemist at Balliol college Oxford.
Learn More About the International Year of the Periodic Table (IYPT) in Crystals Project:
This project (#IYPTCrystals) is part of the International Year of the Periodic Table celebration (#IYPT2019), read more about the project here.
You can follow us on social media; search for #IYPTCrystals or follow The CCDC on X @ccdc_cambridge on Facebook ccdc.cambridge, on Instagram ccdc_cambridge or on YouTube CCDCCambridge.
Understand some of the terms and concepts used with our Frequently Asked Questions page here.
A 3D visualization showing Boron in real crystal structures: