Cadmium
Cadmium:
Cadmium is a bluish silvery, soft, ductile metal, which is a by-product in the production of zinc and other metals. It is very toxic and harmful to the environment. Attribution: Hi-Res Images ofChemical Elements / CC BY (https://creativecommons.org/licenses/by/3.0)
Facts about Cadmium:
- Cadmium: Cadmium is a shiny metal with a bluish cast (shade),very soft.
- Fun fact about Cadmium: In combination with certain metals, it lowers the melting point. Cigarette smoking is a major source of Cadmium.
- Chemical symbol: Cd
- Atomic number: 48
A crystal structure containing Cadmium:
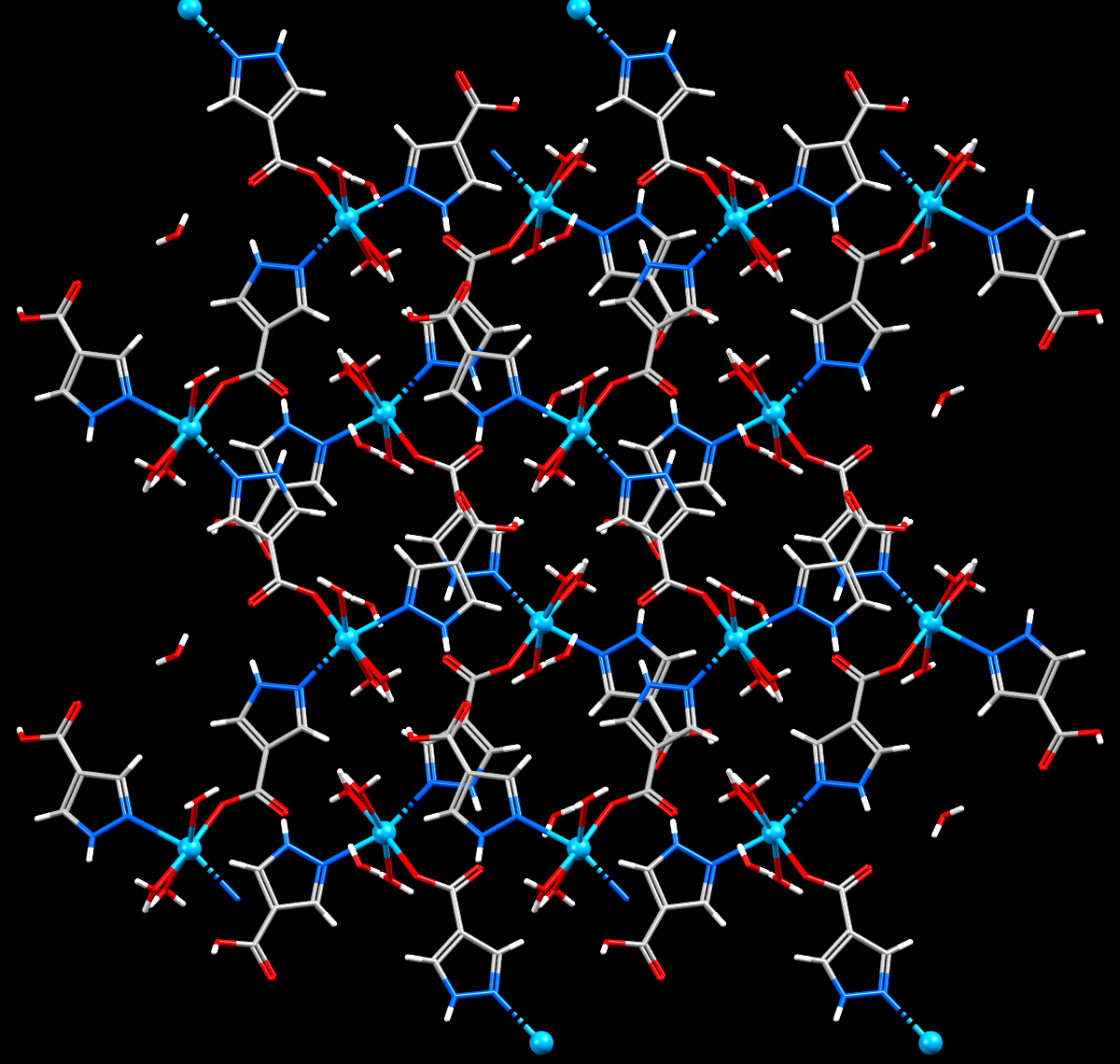
Cadmium displayed as skyblue spheres, is captured inside the channels of a Metal Organic Framework structure.
Facts about this structure:
- Formula: (C8 H12 Cd N4 O7)n, H2O
- Structure name: catena-[bis(μ-1H-pyrazole-4-carboxylato)-tris(aqua)-cadmium(ii) monohydrate]
- Fun fact about the structure: 1D zigzag chain,extending into a 3D structure by hydrogen bonds. As an electrode material for supercapacitors, it exhibits superior capacitive properties with a good rate performance.
- CSD refcode: MUFWES (What’s this?)
- Associated publication: Chao Feng, Guo-Ning Zhang, Chang-Peng Lv, Xiao-Qi Jin, Li-Na Sun, Journal of Inorganic and Organometallic Polymers and Materials, 2019, 29, 1559, DOI: 10.1007/s10904-019-01119-x
More about Cadmium:
Cadmium was discovered simultaneously in 1817 by Friedrich Stromeyer and Karl Samuel Leberecht Hermann, both in Germany, as an impurity in zinc carbonate (calamine). After Stromeyer found the new element as an impurity in calamine, Germany remained the only important producer of the metal for 100 years. The metal was named after the Latin word for calamine, because it was found in this zinc ore. Cadmium has a hexagonal close packed crystalline structure. The first crystal structure of CdI was reported by Richard M. Bozorth as early as 1922. Some examples of applications of Cadmium are batteries, electroplating and anticancer drugs to name a few. A series of Metal Organic Frameworks have been successfully formed to encapsulate Cadmium from water or any other medium, as its presence is hazardous for living bodies. Cadmium is toxic if present above than 0.005mg/l in drinking water according to WHO guidelines.
Learn More About the International Year of the Periodic Table (IYPT) in Crystals Project:
This project (#IYPTCrystals) is part of the International Year of the Periodic Table celebration (#IYPT2019), read more about the project here.
You can follow us on social media; search for #IYPTCrystals or follow The CCDC on X @ccdc_cambridge on Facebook ccdc.cambridge, on Instagram ccdc_cambridge or on YouTube CCDCCambridge.
Understand some of the terms and concepts used with our Frequently Asked Questions page here.
A 3D visualization showing Cadmium in real crystal structures:
