Calcium
Calcium:

A piece of Egyptian alabaster
Facts about Calcium:
- Calcium: Calcium is a silvery metal. It reacts with the air very readily, so the surface quickly becomes dull and tarnished.
- Fun fact about Calcium: Calcium is the most common metal in the human body.
- Chemical symbol: Ca
- Atomic number: 20
A crystal structure containing Calcium:
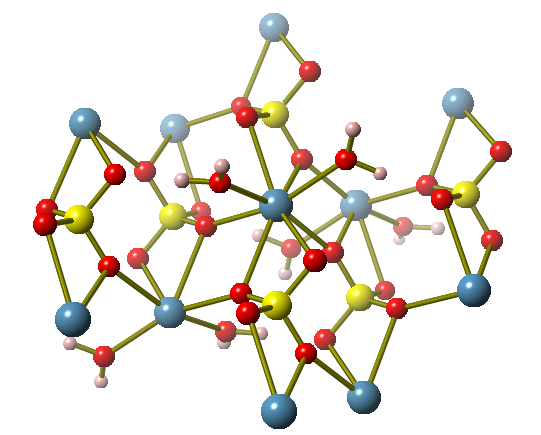
Part of one layer of the crystal structure of gypsum, which is a mineral that contains calcium sulfate. Calcium ions are shown as blue spheres.
Facts about this structure:
- Formula: H4 Ca O6 S
- Structure name: Calcium sulfate(VI) dihydrate
- Fun fact about the structure: Gypsum is made up of layers that contain calcium and sulfate ions. This particular crystal structure used neutron radiation, rather than x-rays.
- ICSD number: 27875 (Find out more about the ICSD database)
- Associated publication: M. Atoji, R.E. Rundle, The Journal of Chemical Physics, 1958, 29, 1306, DOI: 10.1063/1.1744713
More about Calcium:
Calcium occurs in a lot of minerals, such as chalk and limestone. Stalactites and stalagmites in limestone caves are formed when water dissolves the limestone, then evaporates, leaving behind calcium salts and other minerals. Calcium is also found in bones and teeth. Dietary calcium comes from many different foods, including dairy products (e.g. cheese and milk), and leafy green vegetables (e.g. broccoli and cabbage). The Cave of the Crystals in Mexico contains gypsum crystals that are up to 11 m (36 ft) long and 1 m (3 ft) wide!
Learn More About the International Year of the Periodic Table (IYPT) in Crystals Project:
This project (#IYPTCrystals) is part of the International Year of the Periodic Table celebration (#IYPT2019), read more about the project here.
You can follow us on social media; search for #IYPTCrystals or follow The CCDC on X @ccdc_cambridge on Facebook ccdc.cambridge, on Instagram ccdc_cambridge or on YouTube CCDCCambridge.
Understand some of the terms and concepts used with our Frequently Asked Questions page here.
A 3D visualization showing Calcium in real crystal structures: