Curium
Curium:

Curium thermal generators are used to help power space probes, especially when the probe will have no other forms of energy to power them.
Facts about Curium:
- Curium: A shiny metallic element that tarnishes quickly when put in the air.
- Fun fact about Curium: Curium was first revealed publicly after World War II on a children’s radio show before it was officially announced a week later.
- Chemical symbol: Cm
- Atomic number: 96
A crystal structure containing Curium:
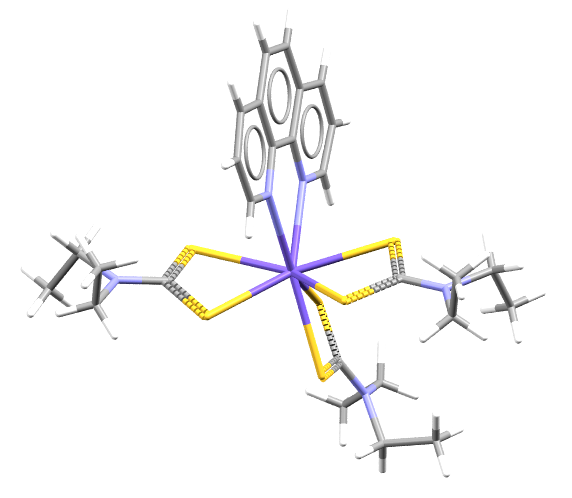
Curium in purple is bonded to organic ligands of Sulfur, in yellow, and Nitrogen, in blue.
Facts about this structure:
- Formula: C27 H38 Cm N5 S6
- Structure name: tris(diethylcarbamodithioato)-(1,10-phenanthroline)-curium(iii)
- Fun fact about the structure: This structure produced pale yellow-orange crystals and enabled the first single crystal X-ray diffraction measurements of Cm(III)-S bond distances.
- CSD refcode: FIHLEQ (What’s this?)
- Associated publication: Samantha K. Cary, Jing Su, Shane S. Galley, Thomas E. Albrecht-Schmitt, Enrique R. Batista, Maryline G. Ferrier, Stosh A. Kozimor, Veronika Mocko, Brian L. Scott, Cayla E. Van Alstine, Frankie D. White, Ping Yang, Dalton Transactions, 2018, 47, 14452, DOI: 10.1039/C8DT02658K
More about Curium:
Curium is an element in the Actinide group of the periodic table with the atomic number 96. It was discovered by Glenn Seaborg, Ralph James, and Albert Ghiorso in 1944, using the cyclotron at Berkeley, California. Curium can be found naturally in very small amounts of uranium and is made by bombarding plutonium with alpha-particles. The elements radioactivity is used in radioisotope thermoelectric generators, which use the heat generated by the radioactive decay to power things that must operate for long periods of time away from contact with people and other sources of energy, such as space probes. Due to its extreme radioactivity, Curium does not have any biological roles but is in fact toxic to living things.
Learn More About the International Year of the Periodic Table (IYPT) in Crystals Project:
This project (#IYPTCrystals) is part of the International Year of the Periodic Table celebration (#IYPT2019), read more about the project here.
You can follow us on social media; search for #IYPTCrystals or follow The CCDC on X @ccdc_cambridge on Facebook ccdc.cambridge, on Instagram ccdc_cambridge or on YouTube CCDCCambridge.
Understand some of the terms and concepts used with our Frequently Asked Questions page here.
A 3D visualization showing Curium in real crystal structures: