Einsteinium
Einsteinium:

The signature ‘mushroom cloud’ arising from the Ivy Mike nuclear test (USA, 1952). Samples collected from this test revealed the existence of Einsteinium. (Photo courtesy: National Nuclear Security Administration/Nevada Field Office)
Facts about Einsteinium:
- Einsteinium: Einsteinium is prepared artificially in laboratories, silvery white in color, metallic and dangerously radioactive!
- Fun fact about Einsteinium: Einsteinium is so highly radioactive that it glows with a visible blue light and the heat arising from this radioactivity destroys the crystal lattice of the element – a phenomenon of self destruction!
- Chemical symbol: Es
- Atomic number: 99
A crystal structure celebrating Einsteinium:
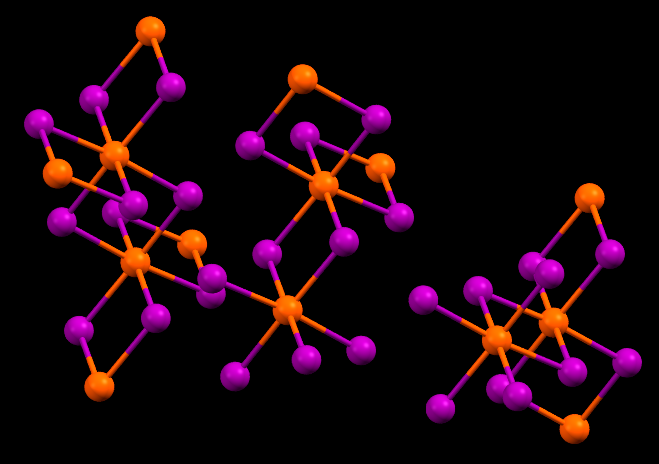
Einsteinium triiodide has the same crystal structure as Bismuth triiodide. Shown here is Bismuth triiodide, with Bismuth atoms marked in orange. Einsteinium triiodide would have Einsteinium atoms at the orange positions.
Facts about this structure:
- Formula: Bi I3
- Structure name: Bismuth(III) iodide
- Fun fact about the structure: Einsteinium triiodide is predicted to have the same crystal structure as this Bismuth triiodide. Einstenium triiodide is amber in color, solid, and glows with a reddish brown in the dark due to the intense radioactivity of Einsteinium
- ICSD refcode: 26083 (Find out more about the ICSD database)
- Associated publication: J. Trotter, T. Zobel, Zeitschrift fur Kristallographie, Kristallgeomie, Krystallphysik, Kristallchemie, 1966, 123, 67 Link to materials project: 10.17188/1199014
More about Einsteinium:
Einsteinium was discovered from the debris of the first experimentally tested hydrogen bomb in 1952. It is perhaps ironic that the namesake of Einsteinium actually warned the scientific community against the very technology that led to the identification of this element. It is a transuranic element, having high radioactivity. The most stable isotope is 253Es, which decays rapidly to 249Bk and 249Cf. Its scarcity (less than few milligrams available from lab scale synthesis every year) and radioactivity renders its chemistry very difficult to explore. Its compounds in the +II and +III oxidation state are often coloured and have beautiful glowing appearance due to its strong radioactivity. Crystal structures of Einsteinium compounds are difficult to determine due to self-destruction of structure caused by the heat accompanying radioactivity. Like other radioactive transuranic elements, Einsteinium also has potential application in cancer therapy if organic motifs for delivering the element to biological sites can be identified. The structure shown in the 3D viewer below is another Bismuth triiodide structure which can be found in the Cambridge Structural Database (CSD) this time due to it’s metal-organic nature. The structure is tetrakis(propan-1-aminium) hexaiodo-bismuth triiodide, the entry in the CSD has CSD Refcode MISBAU and Publication DOI: 10.1039/C8TC04372H.
Learn More About the International Year of the Periodic Table (IYPT) in Crystals Project:
This project (#IYPTCrystals) is part of the International Year of the Periodic Table celebration (#IYPT2019), read more about the project here.
You can follow us on social media; search for #IYPTCrystals or follow The CCDC on X @ccdc_cambridge on Facebook ccdc.cambridge, on Instagram ccdc_cambridge or on YouTube CCDCCambridge.
Understand some of the terms and concepts used with our Frequently Asked Questions page here.
A 3D visualization showing Einsteinium in real crystal structures: