Fluorine
Fluorine:

Teflon, polytetrafluoroethene (or PTFE) is one of the most resilient fluorine compounds, used in non-stick pans and as plumbers tape.
Facts about Fluorine:
- Fluorine: Pale yellow gas at standard conditions
- Fun fact about Fluorine: Fluorine is one of the most reactive and toxic elements – this is because it is the most electron negative element. The only other elements in the periodic table it doesn’t react with are helium and neon.
- Chemical symbol: F
- Atomic number: 9
A crystal structure containing Fluorine:
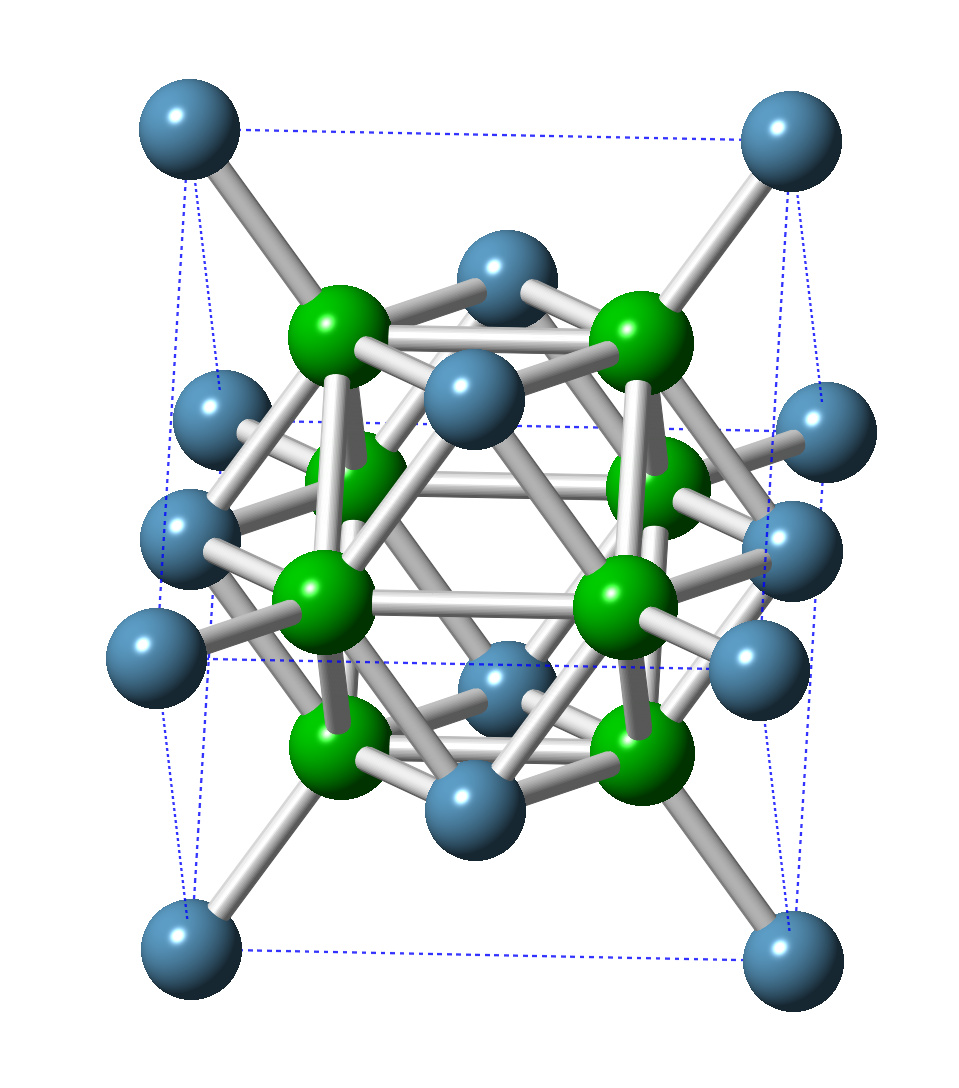
Fluorite is an archetypal structure i.e. many minerals and materials have the same arrangement of atoms. Ca atoms are coloured blue, while the F atoms are in green.
Facts about this structure:
- Formula: CaF2
- Structure name: Fluorite
- Fun fact about the structure: Nearly 500 years ago it was discovered that fluorite (then called fluorspar) would melt and flow when in a fire – it became an additive that made it easier for blacksmiths to work molten metal. It is named after the latin term fluere, meaning “to flow”. The example shown here was subjected to very high pressure for the structure determination.
- ICSD number: 51283 (Find out more about the ICSD database)
- Associated publication: E. Morris, T. Groy, K. Leinenweber, Journal of Physics and Chemistry of Solids, 2001, 62, 1117., DOI: 10.1016/S0022-3697(00)00291-2
More about Fluorine:
While elemental fluorine is highly reactive, its compounds can be quite stable. It was realised centuries ago that fluorides contained an as yet unidentified element similar to chlorine – many scientists died trying to isolate fluorine from these compounds (the fluorine martyrs). Henri Moissan eventually achieved this, for which he got the Nobel Prize in 1906. Many materials adopt the same atomic arrangement as Fluorite and this structure type is fundamental in our understanding of their properties – many fluorites give off light i.e. they are ‘fluorescent’! Fluoride is considered essential for healthy teeth and bones, but only in small amounts, and it is added to drinking water and toothpaste. When you brush your teeth crystals are formed that make them more resistant to acid (which generally makes the tooth porous and prone to decay).
Learn More About the International Year of the Periodic Table (IYPT) in Crystals Project:
This project (#IYPTCrystals) is part of the International Year of the Periodic Table celebration (#IYPT2019), read more about the project here.
You can follow us on social media; search for #IYPTCrystals or follow The CCDC on X @ccdc_cambridge on Facebook ccdc.cambridge, on Instagram ccdc_cambridge or on YouTube CCDCCambridge.
Understand some of the terms and concepts used with our Frequently Asked Questions page here.
A 3D visualization showing Fluorine in real crystal structures: