Lanthanum
Lanthanum:

Rechargeable batteries spelling the letters of the chemical symbol of Lanthanum. The M (for metal) in “NiMH batteries” often stands for La.
Facts about Lanthanum:
- Lanthanum: Silvery-white metal which is very soft (can be cut with knife) and tarnishes slowly when it is exposed to air.
- Fun fact about Lanthanum: Lanthanum is helping us with sustainable transport: both hydrogen-powered and hybrid vehicles use lanthanum compounds. Batteries of a hybrid car can contain several kilograms of La in their anodes!
- Chemical symbol: La
- Atomic number: 57
A crystal structure containing Lanthanum:
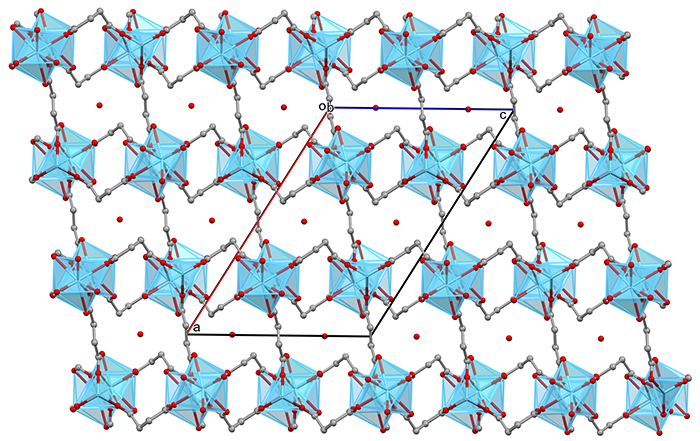
Structure of a Metal Organic Framework material containing La (in the middle of the blue polyhedra), that can be used as a catalyst.
Facts about this structure:
- Formula: (C12 H16 La2 O14)n,n(H2 O)
- Structure name: catena-(tris(μ4-Succinato)-diaqua-di-lanthanum monohydrate)
- Fun fact about the structure: This compound, together with hydrogen peroxide, can oxidise foul-smelling sulfides to sulfoxides and sulfones. After the reaction, it is easily separated by filtration and can be reused several times.
- CSD refcode: GAFXOB (What’s this?)
- Associated publication: J.Perles, M.Iglesias, C.Ruiz-Valero, N.Snejko, Journal of Materials Chemistry, 2004, 14, 2683, DOI: 10.1039/b314220e
More about Lanthanum:
Lanthanum (from the ancient Greek word ‘lanthanein’, to lie hidden) was discovered by Swedish chemist Carl Gustav Mosander, hidden in a cerium mineral. Although initially a shy element, nowadays gives its name to the group of metals with atomic numbers 58-71 (Ce-Lu), called lanthanoids (meaning ‘like lanthanum’). It is not used as a pure element, but you can find La compounds (mostly alloys with other metals) in rechargeable batteries, camera lenses, lighter flints, detectors, studio lighting devices and cinema projectors and also as catalysts in the petroleum industry. La has no known biological role, but lanthanum carbonate can be used as a treatment to lower phosphate levels in the human body.
Learn More About the International Year of the Periodic Table (IYPT) in Crystals Project:
This project (#IYPTCrystals) is part of the International Year of the Periodic Table celebration (#IYPT2019), read more about the project here.
You can follow us on social media; search for #IYPTCrystals or follow The CCDC on X @ccdc_cambridge on Facebook ccdc.cambridge, on Instagram ccdc_cambridge or on YouTube CCDCCambridge.
Understand some of the terms and concepts used with our Frequently Asked Questions page here.
A 3D visualization showing Lanthanum in real crystal structures: