Osmium
Osmium:

Metallic osmium in the form of crystalline clusters which were grown by chemical vapor transport
Facts about Osmium:
- Osmium: A lustrous bluish-white transition metal that is hard, but brittle.
- Fun fact about Osmium: Osmium is twice as dense as lead and is the most dense naturally occurring element.
- Chemical symbol: Os
- Atomic number: 76
A crystal structure containing Osmium:
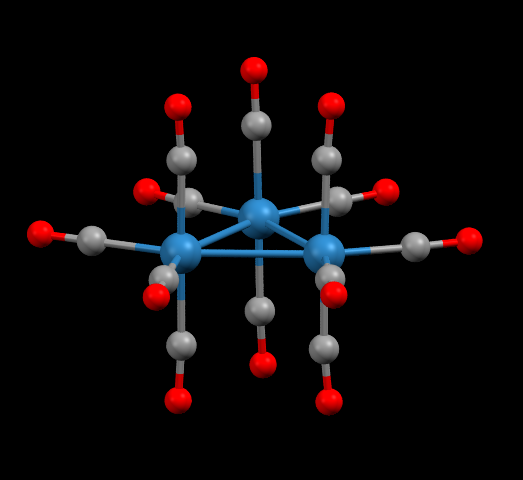
Os3(CO)12 is an important metal-organic molecule that has been used to make hundreds of other osmium compounds
Facts about this structure:
- Formula: Os3(CO)12
- Structure name:dodecacarbonyl-triangulo-triosmium
- Fun fact about the structure: The osmium atoms in this structure form an equilateral triangle. Four carbon monoxide groups are attached to each osmium atom.
- CSD refcode: FOKBUC01 (What’s this?)
- Associated publication: M.R.Churchill, B.G.DeBoer, Inorganic Chemistry, 1977, 16, 878., DOI: 10.1021/ic50170a032
More about Osmium:
The name of the element osmium is derived from the Greek word ‘osme’ which means ‘smell’ because of the ashy and smoky smell of osmium tetroxide. Osmium is the least abundant stable element in the Earth’s crust and is therefore very expensive. It is an exceptionally hard durable metal and has been used in alloys for small items such as electrical contacts, nibs of fountain pens and instrument pivots. Some osmium compounds are useful catalysts for a variety of reactions and some have been shown to exhibit anti-cancer activity. Osmium has no known biological role and is not toxic in elemental form but its oxide, osmium tetroxide, is an extremely toxic and volatile solid.
Learn More About the International Year of the Periodic Table (IYPT) in Crystals Project:
This project (#IYPTCrystals) is part of the International Year of the Periodic Table celebration (#IYPT2019), read more about the project here.
You can follow us on social media; search for #IYPTCrystals or follow The CCDC on X @ccdc_cambridge on Facebook ccdc.cambridge, on Instagram ccdc_cambridge or on YouTube CCDCCambridge.
Understand some of the terms and concepts used with our Frequently Asked Questions page here.
A 3D visualization showing Osmium in real crystal structures: