Protactinium
Protactinium:
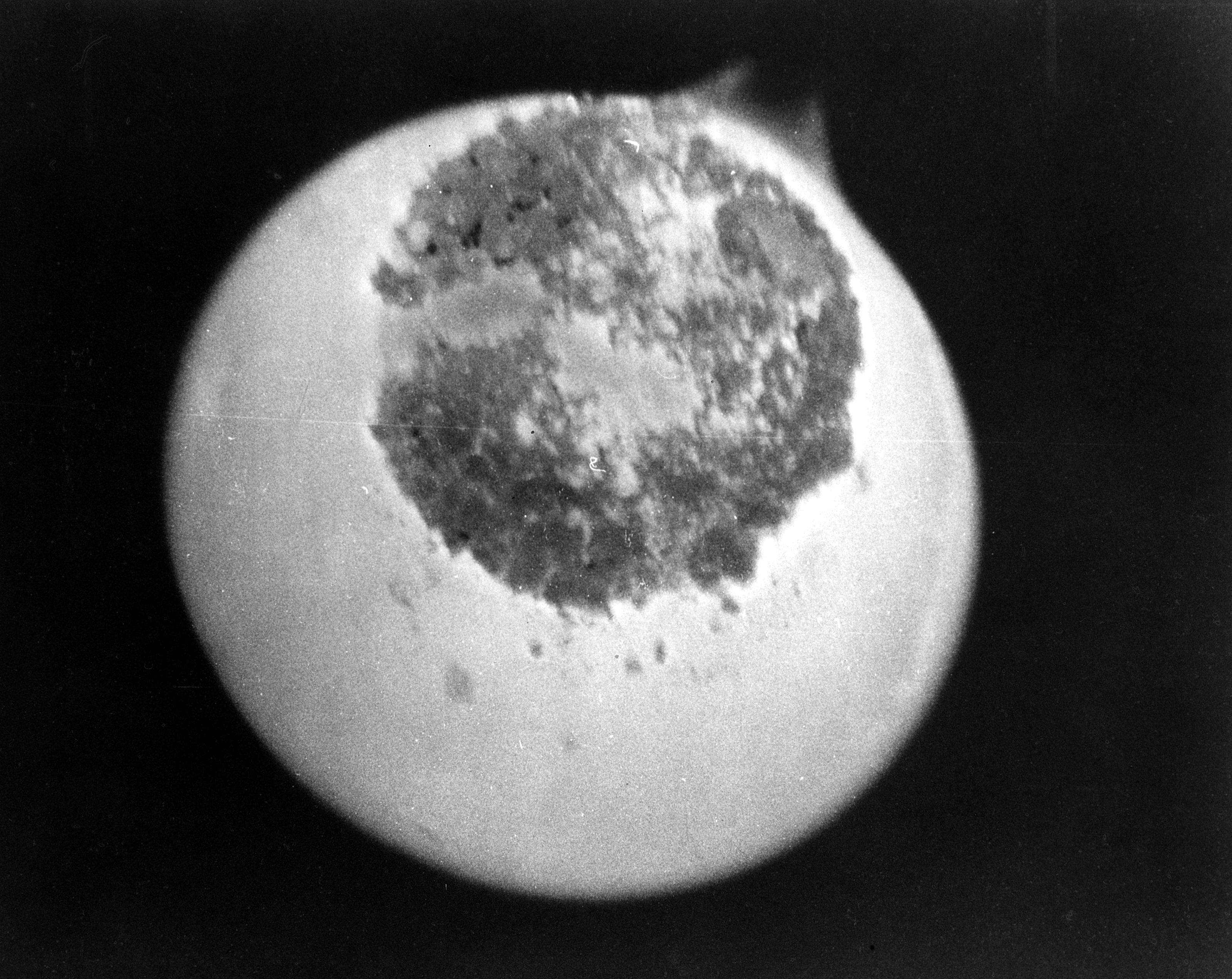
Photograph of protactinium-233 (darker circular area) taken at the National Reactor Testing Station in Idaho circa 1969. The bright area is the light emanating from its radioactivity, which was used to photograph this rare image.
Facts about Protactinium:
- Protactinium: Dense, silvery-grey, radioactive metal
- Fun fact about Protactinium: As the 5f and 6d orbitals switch filling order at protactinium, understanding the chemistry of this rare element could provide an insight into the interplay between relativistic effects and bonding behaviour.
- Chemical symbol: Pa
- Atomic number: 91
A crystal structure containing Protactinium:
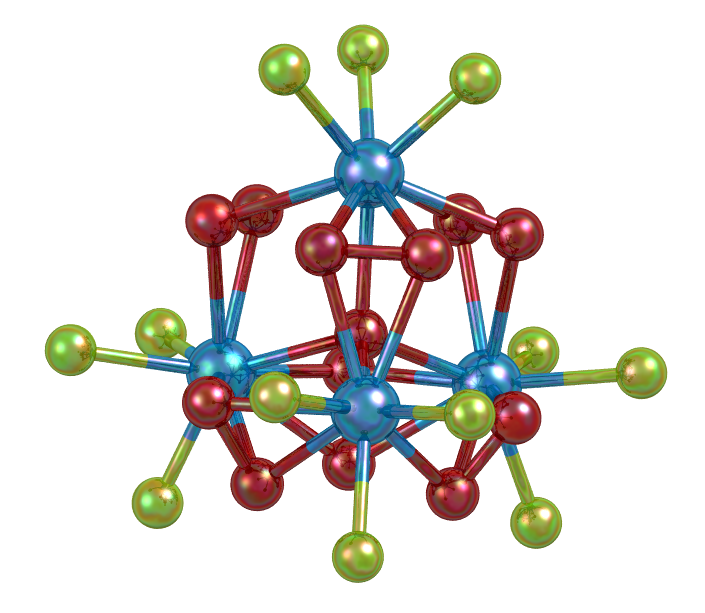
Image showing the tetranuclear protactinium peroxo-cluster [Pa4O(O2)6F12]6- in A6[Pa4O(O2)6F12] where A = Rb+, Cs+, (CH3)4N+
Facts about this structure:
- Formula: F12 O13 Pa4 6-,7(C4 H12 N +),15(H2 O),F –
- Structure name: heptakis(tetramethylammonium) (μ-oxo)-hexakis(μ-peroxo)-dodecafluoro-tetra-protactinium(v) fluoride pentadecahydrate
- Fun fact about the structure: This structure is the first example of a tetranuclear protactinium peroxo-cluster and demonstrates a marked difference in bonding behaviour between protactinium and uranium.
- CSD refcode: BEPWAX (What’s this?)
- Associated publication: Richard E. Wilson, Stephanie De Sio, Valérie Vallet, Nature Communications, 2018, 9, 622, DOI: 10.1038/s41467-018-02972-z
More about Protactinium:
Protactinium is one of the rarest naturally occuring elements with an approximate abundance in the earth’s crust of 1.4 parts per trillion. Originally named “brevium” by Kasimar Fajans and Oswald Helmut Göhring from the Latin word brevis meaning brief, due to the very short half life of the protactinium-234 isotope they observed, the IUPAC name “protactinium” was chosen after Otto Hahn and Lise Meitner discovered the more stable protactinium-231 isotope. This name was derived from the Greek word protos meaning first or before, therefore the name protactinium refers to this element being the parent of actinium in the nuclear decay chain of uranium-235. There are 29 known radioisotopes of protactinium with half lifes ranging from 53 nanoseconds for protactinium-219 to 32,760 years for protactinium-231. Protactinium primarily exhibits the +4 and +5 oxidation states (although +2 and +3 are also possible) and forms a variety of inorganic and organometallic compounds. Protactinium-231 is also used in conjunction with thorium-230 as a double-isotope tracer for radiometric dating, particularly for marine sediment.
Learn More About the International Year of the Periodic Table (IYPT) in Crystals Project:
This project (#IYPTCrystals) is part of the International Year of the Periodic Table celebration (#IYPT2019), read more about the project here.
You can follow us on social media; search for #IYPTCrystals or follow The CCDC on X @ccdc_cambridge on Facebook ccdc.cambridge, on Instagram ccdc_cambridge or on YouTube CCDCCambridge.
Understand some of the terms and concepts used with our Frequently Asked Questions page here.
A 3D visualization showing Protactinium in real crystal structures: