Seaborgium
Seaborgium:
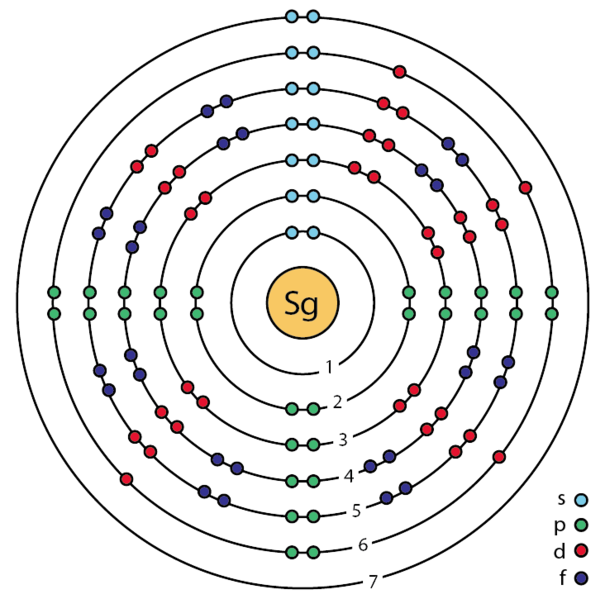
The electronic configuration of an atom of Seaborgium. (from Wikipedia)
Facts about Seaborgium:
- Seaborgium: Seaborgium is a synthetic element so its properties are unknown, but it is expected to behave like Molybdenum and Tungsten.
- Fun fact about Seaborgium: The element Seaborgium was named for Glenn T. Seaborg who helped first discover and synthesize it. It was the first element named after a living person which was very controversal at the time.
- Chemical symbol: Sg
- Atomic number: 106
A crystal structure celebrating Seaborgium:
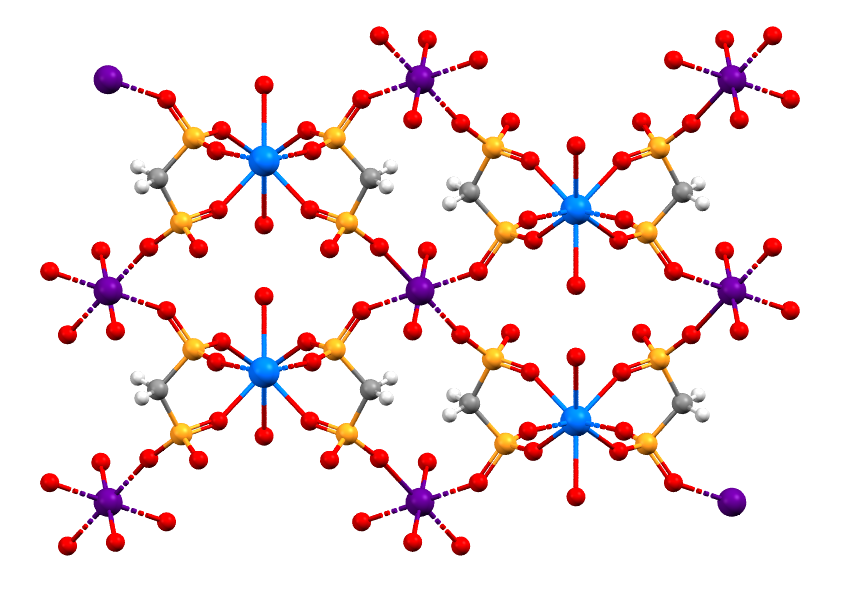
This structure contains two actinide elements, Uranium (purple) and Plutonium (blue), as well as Carbon (grey), Oxygen (red), Phosphorus (orange), and Hydrogen (white).
Facts about this structure:
- Formula: (C2 H10 O16 P4 Pu U)n
- Structure name: catena-[bis(μ4-Hydrogen methylenediphosphonato-O,O’,O”,O”’,O””)-diaqua-dioxo-[242Pu]plutonium(iv)-uranium(vi)]
- Fun fact about the structure: This structure contains two actinide elements, Uranium and Plutonium, which Glenn Seaborg helped to discover. Through his research Seaborg and co-workers worked to discover ten heavy elements, and helped to arrange the actinide series in the Periodic Table
- CSD refcode: DUGSEE (What’s this?)
- Associated publication: A.-G.D.Nelson, T.H.Bray, F.A.Stanley, T.E.Albrecht-Schmitt, Inorganic Chemistry, 2009, 48, 4530, DOI: 10.1021/ic900484w
More about Seaborgium:
Seaborgium is expected to behave like other members of Group 6 in the Periodic Table; Molybdenum and Tungsten. Experiments done with compounds of Seaborgium confirm it behaves like other members of Group 6. Seaborgium is radioactive with a very short half life and only a very small amount of Seaborgium has been made. To date it has been used only for scientific study.
Learn More About the International Year of the Periodic Table (IYPT) in Crystals Project:
This project (#IYPTCrystals) is part of the International Year of the Periodic Table celebration (#IYPT2019), read more about the project here.
You can follow us on social media; search for #IYPTCrystals or follow The CCDC on X @ccdc_cambridge on Facebook ccdc.cambridge, on Instagram ccdc_cambridge or on YouTube CCDCCambridge.
Understand some of the terms and concepts used with our Frequently Asked Questions page here.
A 3D visualization showing Seaborgium in real crystal structures: