Selenium
Selenium:

Selenium functions as a critical regulator of vital metabolic and physiological pathways involved in the aging process.
Facts about Selenium:
- Selenium: Selenium exists in a number of allotropic forms. The amorphous forms of selenium do not have specific melting points. Selenium is a fairly reactive element.
- Fun fact about Selenium: Selenium gets its name from the Greek word “selene”, which means “moon”. Selene was the Greek goddess of the moon.
- Chemical symbol: Se
- Atomic number: 34
A crystal structure containing Selenium:
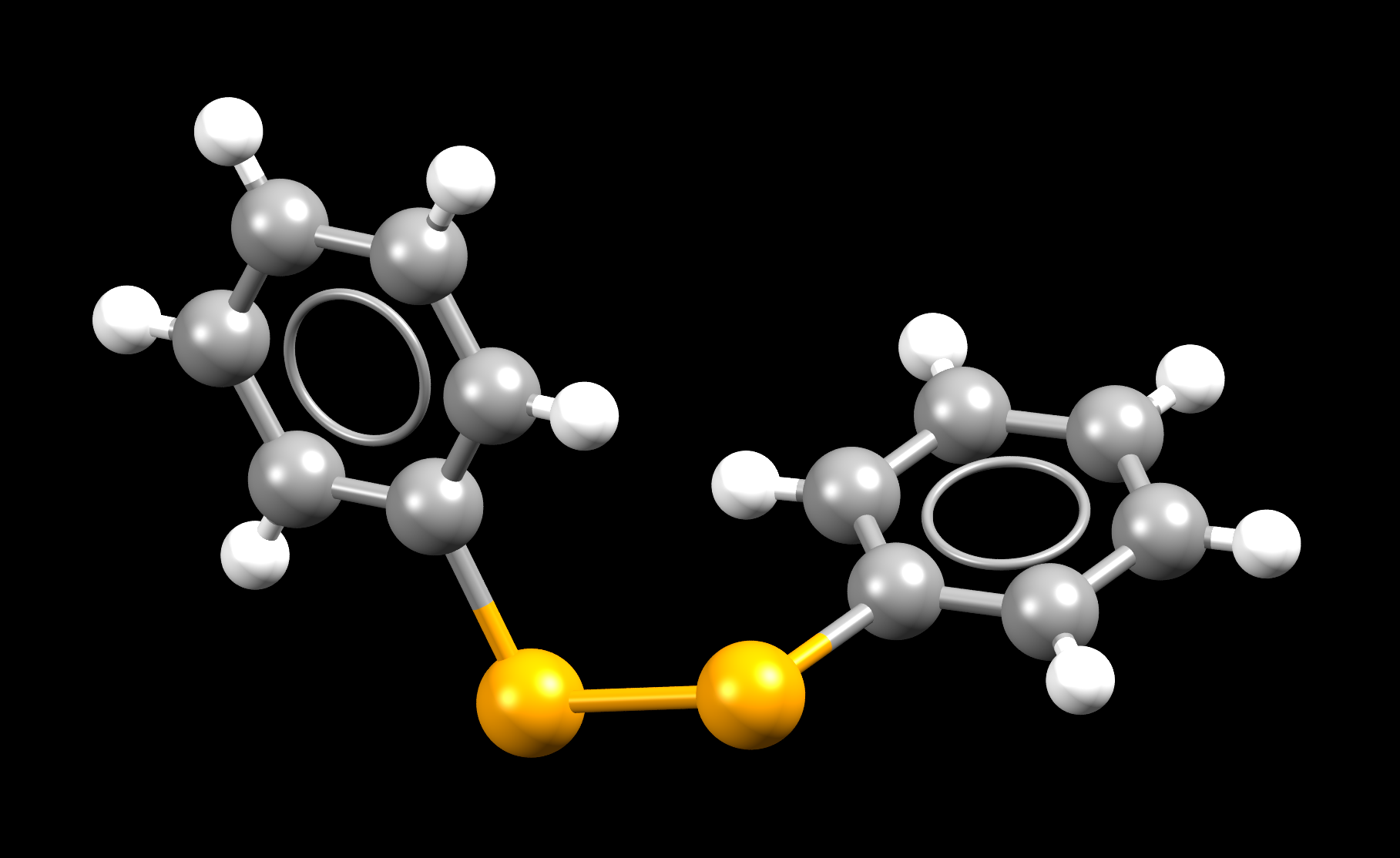
Hydrogen atoms bound to carbon were idealised. Colour code is; C-grey, H-white, Se-orange. Structural and computational studies suggest that the mirror image (the (P)- enantiomer) of this molecule is energetically indistinguishable from this one (the M-enantiomer).
Facts about this structure:
- Formula: C12 H10 Se2
- Structure name: (M)-Diphenyl diselenide
- Fun fact about the structure: Selenium compounds are considered “Janus compounds”, i.e., products with a double face, because of their contrasting behavior that is dose-dependent.
- CSD refcode: DPHDSE02 (What’s this?)
- Associated publication: A.L.Fuller, L.A.S.Scott-Hayward, Yang Li, M.Buhl, A.M.Z.Slawin, J.D.Woollins, Journal of the American Chemical Society, 2010, 132, 5799, DOI: 10.1021/ja100247y
More about Selenium:
Selenium is a member of the group 16 elements (O, S, Se, Te and radioactive Po), collectively known as chalcogens. It was discovered in 1817 by J.J.Berzellius in the reddish deposits that formed in the lead chambers at his sulfuric acid plant at Gripsholm in Sweden. Selenium has several allotropic forms in both the amorphous and crystalline states at room temperature and adopts either a helical polymer chain or Se8 ring structures with Se-Se distances varying between 2.32 and 2.37Å. The following three major, interdependent factors have contributed to this rapid development of the field:
i) role in organic chemistry: Selenium can be introduced to a myriad of organic substrates as an electrophile, nucleophile or even as a radical in a chemo-, regio-, and stereo-selective manner
ii) organometallic chemistry and materials science: metal selenolates have emerged as versatile single-source molecular precursors for the synthesis of nanoparticles and deposition of thin films of metal-selenides.
iii) Selenium in biology: selenium was long considered a poison until 1957 when Schwarz and Foltz identified it as an essential micronutrient.
Learn More About the International Year of the Periodic Table (IYPT) in Crystals Project:
This project (#IYPTCrystals) is part of the International Year of the Periodic Table celebration (#IYPT2019), read more about the project here.
You can follow us on social media; search for #IYPTCrystals or follow The CCDC on X @ccdc_cambridge on Facebook ccdc.cambridge, on Instagram ccdc_cambridge or on YouTube CCDCCambridge.
Understand some of the terms and concepts used with our Frequently Asked Questions page here.
A 3D visualization showing Selenium in real crystal structures: