Strontium
Strontium:

An image of red fireworks. Strontium burns red in air and strontium-containing salts are added to fireworks to produce a red colour.
Facts about Strontium:
- Strontium: Silvery white metal at room temperature
- Fun fact about Strontium: Strontium can take the place of calcium in bones as the atoms are a similar size.
- Chemical symbol: Sr
- Atomic number: 38
A crystal structure containing Strontium:
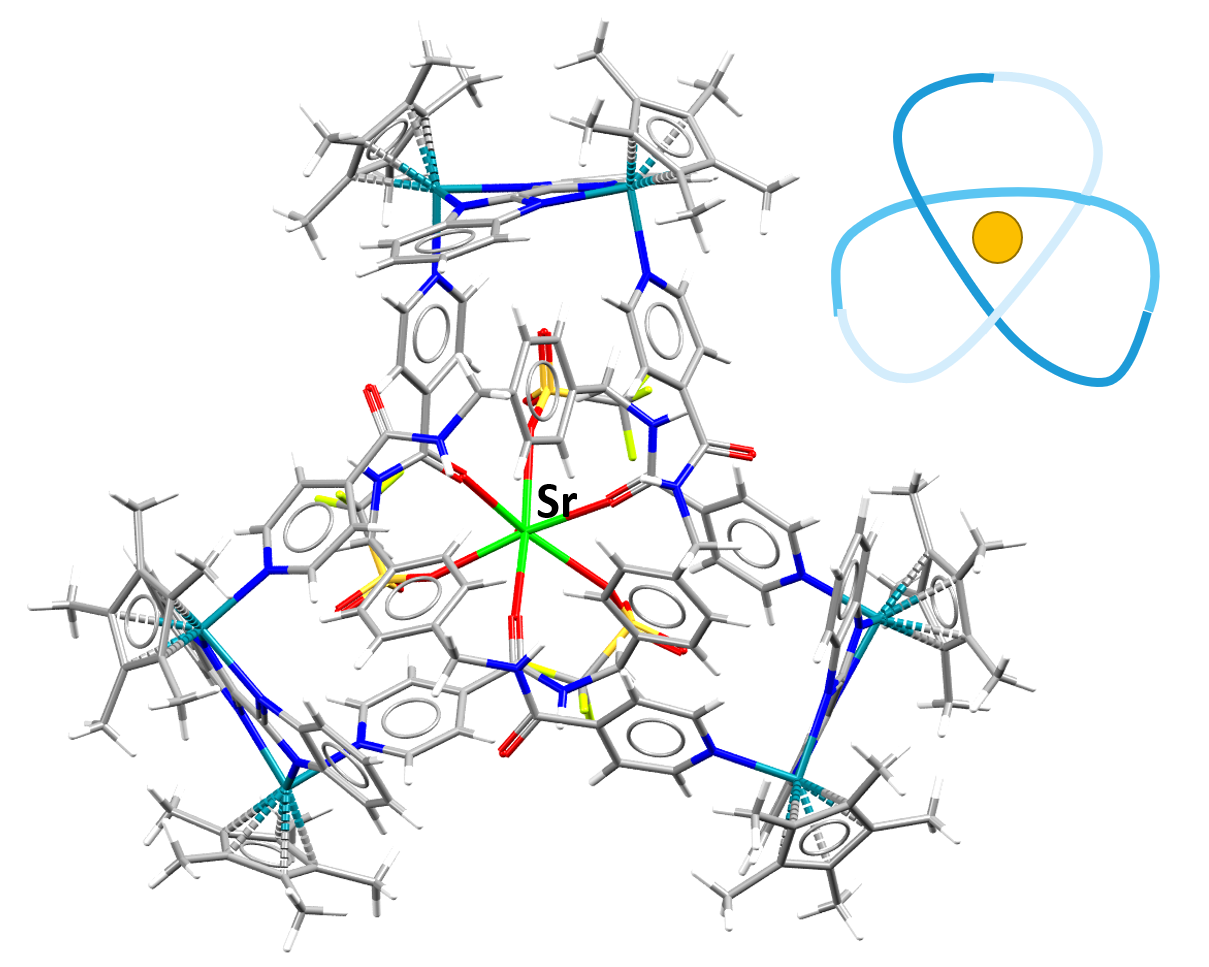
Image showing a Strontium-containing compound in a trefoil knot formation. The line drawing in the top right hand corner illustrates how the atoms are arranged in the knot.
Facts about this structure:
- Formula: C165 H170 F9 N24 O16 Rh6 S3 Sr 5+,5(C F3 O3 S –),3.5(H2 O)
- Structure name: tris(μ-1H,1’H-2,2′-bibenzimidazole)-tris(μ-N,N’-[1,4-phenylenebis(methylene)]bis(pyridine-4-carboxamide))-aqua-(pentamethyl-cyclopentadienyl)-tris(trifluoromethanesulfonato)-hexa-rhodium-strontium pentakis(trifluoromethanesulfonate) hydrate
- Fun fact about the structure: The addition of Strontium causes the formation of the trefoil knot in this structure. This process can be reversed and the removal of strontium causes a figure-eight knot to form.
- CSD refcode: ZUBPOE (What’s this?)
- Associated publication: Li-long Dang, Xiang Gao, Yue-Jian Lin, Guo-Xin Jin, Chemical Science, 2020, 11, 1226, DOI: 10.1039/C9SC05796J
More about Strontium:
Strontium belongs to the alkaline earth metal group of the periodic table. It is a highly reactive element and reacts strongly with both water and air.
This element is named after the village of Strontian in Scotland, near to where it was first discovered. Previously, Strontium was used in the production of sugar from sugar beet via the ‘strontian process’, however, cheaper methods are now more commonly used. Strontium was also used in the manufacture of older colour televisions, where it was added to the glass to prevent the emission of X-rays from the cathode-ray tubes that were used to produce the images.
A more modern use of strontium is in the manufacture of glow in the dark toys. When small amounts of another element, such as Europium, are added to Strontium Aluminate (SrAl2O4) the material becomes phosphorescent – meaning the compound can give off light after it has absorbed radiation. This glowing effect can last up to 14 hours!
Strontium is also used in medicine, due to its similar size to Calcium. A radioactive isotope of Strontium (89Sr) is used to treat bone cancer because of its short half-life (~ 50 days).
Learn More About the International Year of the Periodic Table (IYPT) in Crystals Project:
This project (#IYPTCrystals) is part of the International Year of the Periodic Table celebration (#IYPT2019), read more about the project here.
You can follow us on social media; search for #IYPTCrystals or follow The CCDC on X @ccdc_cambridge on Facebook ccdc.cambridge, on Instagram ccdc_cambridge or on YouTube CCDCCambridge.
Understand some of the terms and concepts used with our Frequently Asked Questions page here.
A 3D visualization showing Strontium in real crystal structures: