Tantalum
Tantalum:

Tantalum is used to make high-performance capacitors. They are small but have high capacitance, and used in smart phones, PC’s and cars, among other things.
Facts about Tantalum:
- Tantalum: Gray, ductile metal of high melting point (2996°C) and very resistant to acids.
- Fun fact about Tantalum: Named after Tantalus of Greek mythology. As the name indicates, it has a tantalizing chemistry.
- Chemical symbol: Ta
- Atomic number: 73
A crystal structure containing Tantalum:
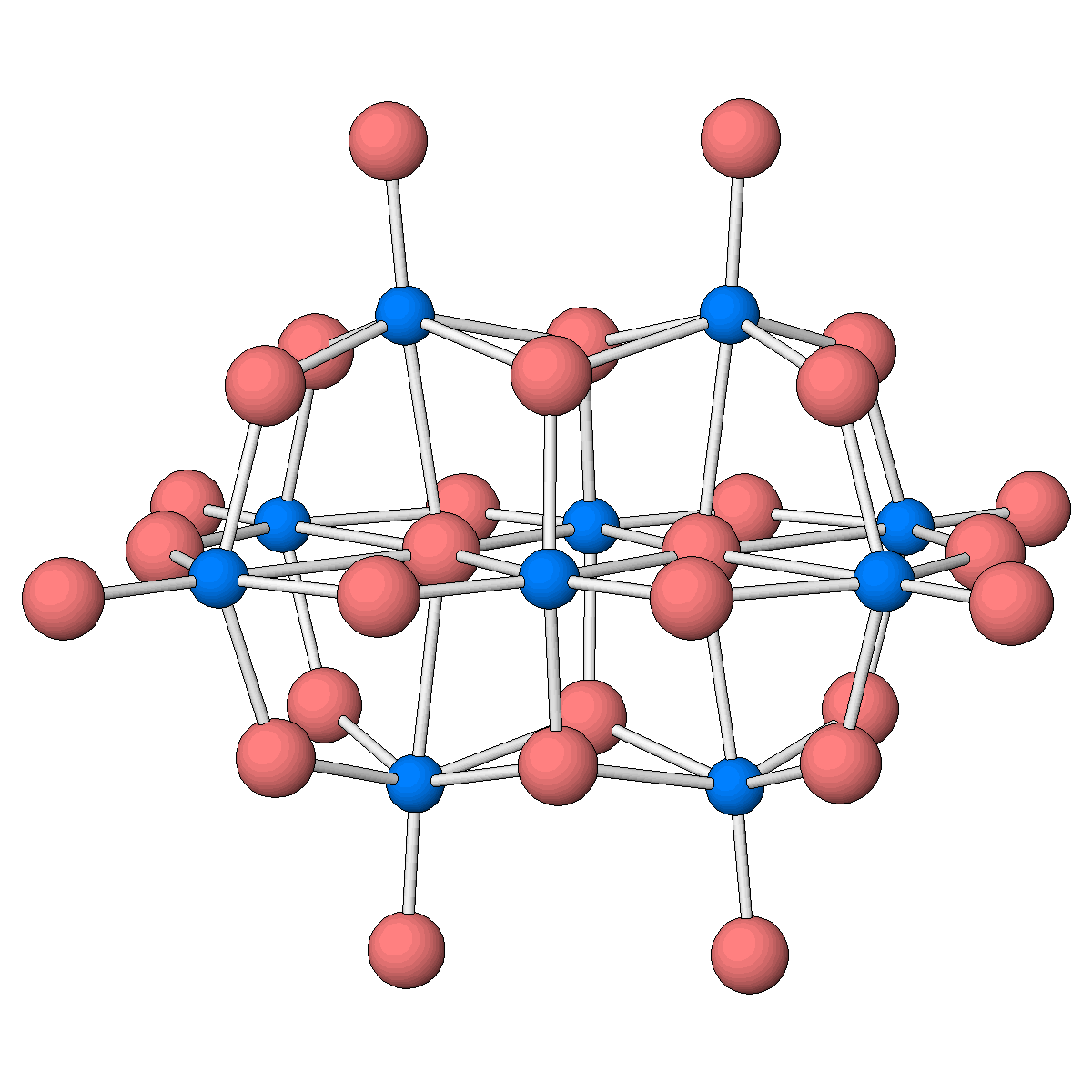
Structure of the decatantalate anion, one of only two molecular oxides of tantalum known to date.
Facts about this structure:
- Formula: O28 Ta10 6-,6(H2 O),6(C16 H36 N +)
- Structure name: hexakis(tetrabutylammonium) bis(μ4-oxo)-tetrakis(μ3-oxo)-tetradecakis(μ2-oxo)-octaoxo-deca-tantalum hexahydrate
- Fun fact about the structure: Although the vanadium and niobium analogues were structurally characterized almost half a century ago, the decatantalate had long been missing.
- CSD refcode: WIQPET (What’s this?)
- Associated publication: Miki Matsumoto, Yoshiki Ozawa, Atsushi Yagasaki, Yang Zhe, Inorganic Chemistry, 2013, 52, 7825, DOI: 10.1021/ic400864e
More about Tantalum:
Tantalum is a very unreactive metal and insoluble in acids, even in aqua regia. Its high melting point is exceeded only by tungsten, rhenium, osmium and carbon. Its considerable chemical stability makes tantalum suitable for the manufacture of apparatus used in chemical experiments such as spatulas and crucibles, and of dental and surgical instruments (pins for bones, prostheses, clamps, screws for jaws). Although the metal and oxides have virtually no solution chemistry, tetrabutylammonium salts of polytantalates are extremely hygroscopic.
Learn More About the International Year of the Periodic Table (IYPT) in Crystals Project:
This project (#IYPTCrystals) is part of the International Year of the Periodic Table celebration (#IYPT2019), read more about the project here.
You can follow us on social media; search for #IYPTCrystals or follow The CCDC on X @ccdc_cambridge on Facebook ccdc.cambridge, on Instagram ccdc_cambridge or on YouTube CCDCCambridge.
Understand some of the terms and concepts used with our Frequently Asked Questions page here.
A 3D visualization showing Tantalum in real crystal structures: