Thorium
Thorium:

Image of camera lenses. The yellowed lens is due to thorium radioactivity and can be reversed by exposing the lens to UV light. CC BY-SA 2.0.
Facts about Thorium:
- Thorium: Thorium is a soft, silvery-white metal, solid at room temperature.
- Fun fact about Thorium: Thorium is named after the Norse god of thunder, Thor, even though Thor’s hammer is not made of the element.
- Chemical symbol: Th
- Atomic number: 90
A crystal structure containing Thorium:
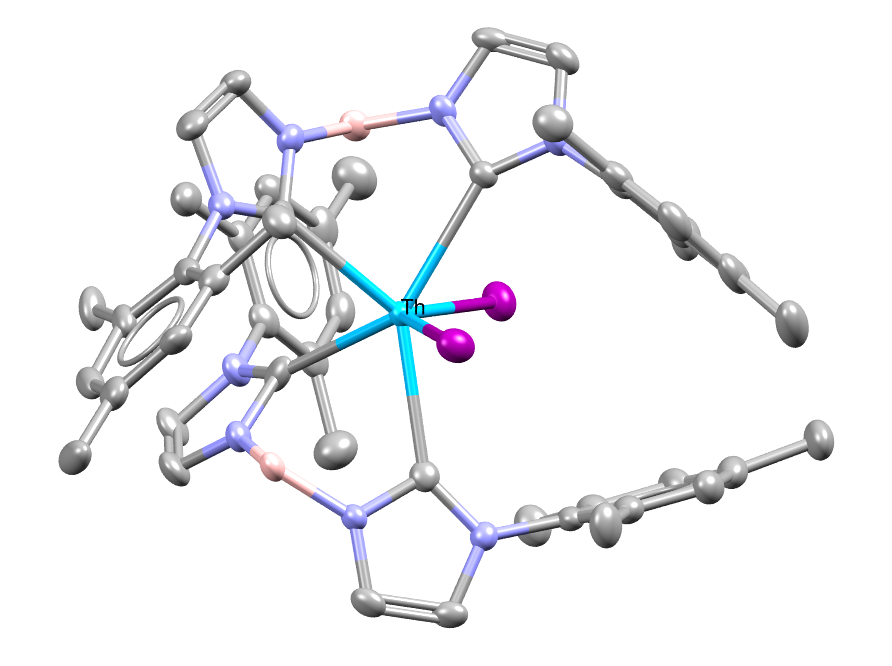
A thorium-based complex with small NHC supporting ligands. The complex provides new information on actinide chemistry. Hydrogen atoms omitted for clarity.
Facts about this structure:
- Formula: C48 H56 B2 I2 N8 Th
- Structure name: bis(Iodo)-bis(μ-1,1′-dihydridoboranidylidenebis(3-mesityl-2,3-dihydro-1H-imidazol-2-ylidene))-thorium
- Fun fact about the structure: Actinide-NHC complexes are rare, thus the thorium based NHC complex in this work is only the second set of Th-NHC complex published.
- CSD refcode: OWAVEP (What’s this?)
- Associated publication: Mary E. Garner, Stephan Hohloch, Laurent Maron, John Arnold, Organometallics, 2016, 35, 2915, DOI: 10.1021/acs.organomet.6b00467
More about Thorium:
Thorium was discovered in 1829 by Swedish chemist Jöns Jacob Berzelius in a sample from a mineral eventually named Thorite by Berzelius. The mineral thorite was found in Norway by Morten Thrane Esmark, a Norwegian priest and amateur mineralogist. Both the mineral and element are named after the Norse god of thunder, Thor. Earlier uses of thorium include as a light source, by heating thorium oxide, in the production of ceramics and carbon arc lamps. The radioactive nature of thorium was first observed in 1898. A better understanding of the biological effects of radiation led to banning some uses such as in treatment of diabetes and rheumatism. Thorium is still used as an alloying element for magnesium while thorium oxide is used in catalysis, in the production of high-quality camera lenses and high temperature crucibles. There is interest in using thorium as an energy source in the form of nuclear fuel. Although not fissile, thorium transmutes to uranium-238 upon absorbing a neutron.
Learn More About the International Year of the Periodic Table (IYPT) in Crystals Project:
This project (#IYPTCrystals) is part of the International Year of the Periodic Table celebration (#IYPT2019), read more about the project here.
You can follow us on social media; search for #IYPTCrystals or follow The CCDC on X @ccdc_cambridge on Facebook ccdc.cambridge, on Instagram ccdc_cambridge or on YouTube CCDCCambridge.
Understand some of the terms and concepts used with our Frequently Asked Questions page here.
A 3D visualization showing Thorium in a real crystal structure: