Tin
Tin:

Droplet of solidified molten tin
Facts about Tin:
- Tin: A soft, pliable, silvery metal that characteristically has a faint yellow hue.
- Fun fact about Tin: Tin has been used for thousands of years, since the Bronze Age. It is commonly associated with packaging such as tin cans and corrosion resistant plating of steel.
- Chemical symbol: Sn
- Atomic number: 50
A crystal structure containing Tin:
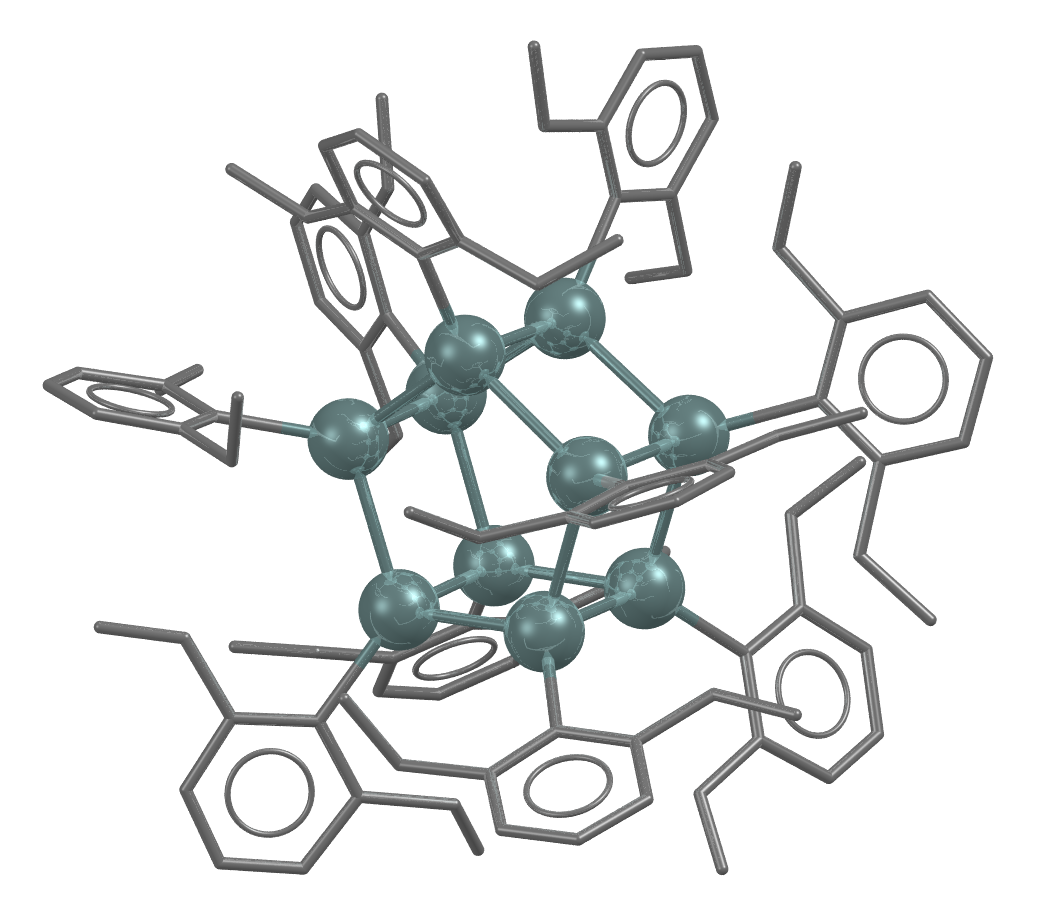
Structure of a tin prismane, with tin atoms shown as teal coloured spheres
Facts about this structure:
- Formula: C100 H130 Sn10,x(C7 H14),x(C7 H8)
- Structure name: decakis(2,6-Diethylphenyl)-decastanna(5)prismane methylcyclohexane toluene solvate
- Fun fact about the structure: Published in 1991 by L. Sita and I. Kinoshita this was the first structure with a heavy atom double pentane centre, known as [5]Prismane or Pentaprismane
- CSD refcode: JIMFUG (What’s this?)
- Associated publication: L.R.Sita, I.Kinoshita, Journal of the American Chemical Society, 1991, 113, 1856. DOI: 10.1021/ja00005a074
More about Tin:
Tin has been extracted from ore since the beginning of the Bronze Age, about 3000 years B.C., most commonly from its ore Cassiterite. The Latin name “Stannum” came to mean Tin in the 4th century. Tin reacts with acids and alkalis but resists corrosion from water. Tin is used as a protective coating for other metals as a protective oxide layer prevents further oxidation. Tin can be highly polished. Treatment of window glass with salts of tin produces electrically conductive coatings, such as those allowing the glass to be switched into opaque mode.
Learn More About the International Year of the Periodic Table (IYPT) in Crystals Project:
This project (#IYPTCrystals) is part of the International Year of the Periodic Table celebration (#IYPT2019), read more about the project here.
You can follow us on social media; search for #IYPTCrystals or follow The CCDC on X @ccdc_cambridge on Facebook ccdc.cambridge, on Instagram ccdc_cambridge or on YouTube CCDCCambridge.
Understand some of the terms and concepts used with our Frequently Asked Questions page here.
A 3D visualization showing Tin in a real crystal structure: