Tungsten
Tungsten:

Image of light bulb filament made of tungsten alloy.
Facts about Tungsten:
- Tungsten: Grayish white, lustrous solid
- Fun fact about Tungsten: Tungsten has the highest melting point of all discovered elements, melting at 3422°C (6192°F). It also has the highest boiling point, at 5930°C (10706°F) and and the highest tensile strength.
- Chemical symbol: W
- Atomic number: 74
A crystal structure containing Tungsten:
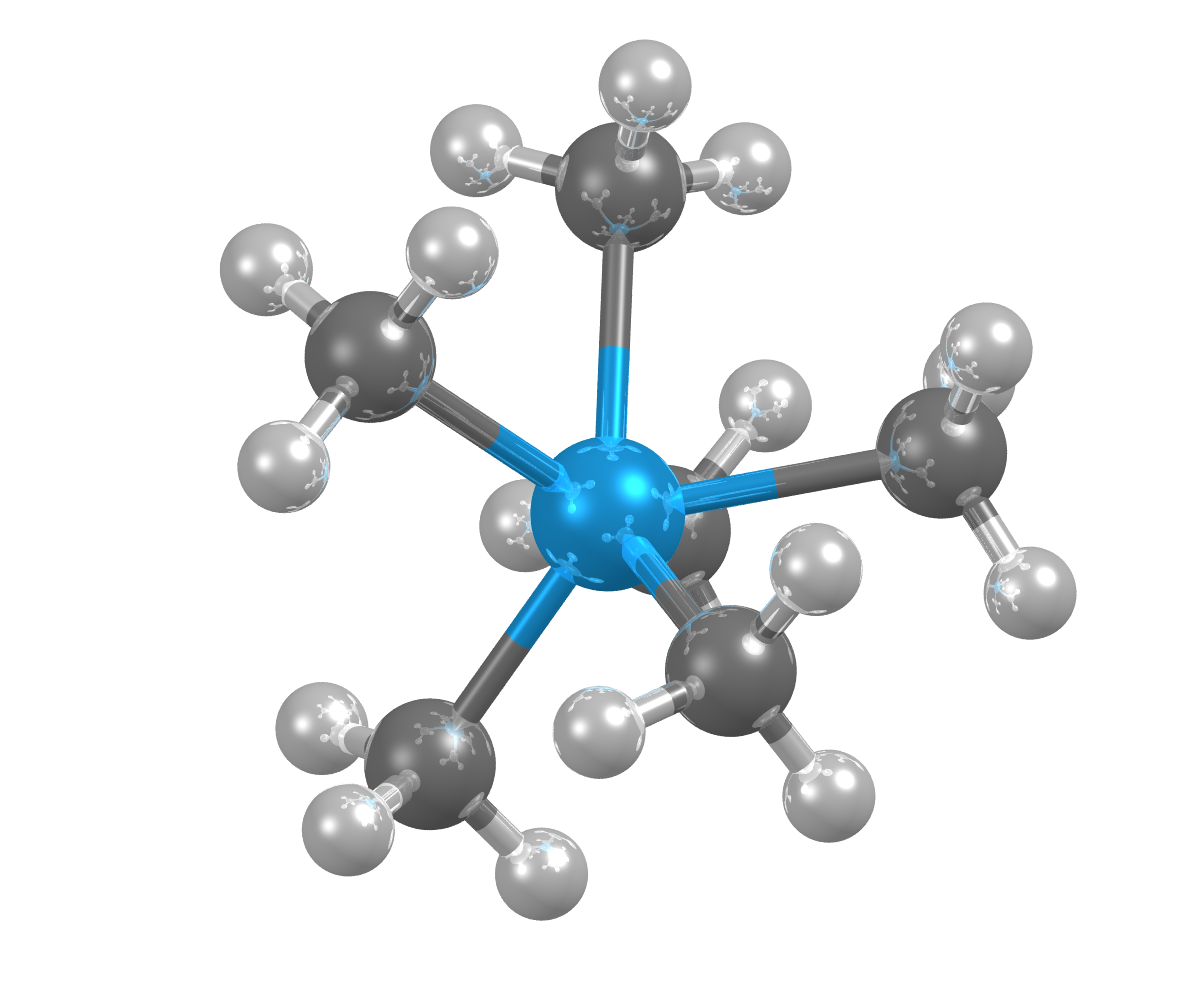
Image showing the crystal structures of hexamethyl-tungsten, which can be used in the manufacture of semiconductor devices. Tungsten shown as blue sphere.
Facts about this structure:
- Formula: C6 H18 W
- Structure name: Hexamethyl-tungsten
- Fun fact about the structure: This structure was determined by x-ray single-crystal diffraction at -163°C, because hexamethyl-tungsten decomposes at room temperature.
- CSD refcode: ZOSXEK (What’s this?)
- Associated publication: V.Pfennig, K.Seppelt, Science, 1996, 271, 626., DOI: 10.1126/science.271.5249.626
More about Tungsten:
Tungsten’s element symbol W was derived from an old name of the ore, Wolframite. However, it was called tungsten (Swedish for “heavy stone”) in English. The name Wolfram was dropped in 2005, to make the periodic table standard. This is probably one of the most highly disputed name changes in the periodic table. It may develop a rainbow oxide when exposed to air. It is the 4th hardest element after carbon, boron, and chromium. Tungsten is one of five refractory metals, meaning it exhibits high resistance to heat and wear. It is the heaviest element used in biochemical reactions; certain bacteria use it in an enzyme. Natural tungsten consists of five stable isotopes with radioactive decay half-life of four quintillion years. The military uses tungsten to make bullets and missiles used in “kinetic bombardment”, which uses a dense material to breach armour instead of explosives. Tungsten has been found in counterfeit gold bricks, because its density is similar to that of gold. The ball in the Ballpoint pen is usually made of Tungsten.
Learn More About the International Year of the Periodic Table (IYPT) in Crystals Project:
This project (#IYPTCrystals) is part of the International Year of the Periodic Table celebration (#IYPT2019), read more about the project here.
You can follow us on social media; search for #IYPTCrystals or follow The CCDC on X @ccdc_cambridge on Facebook ccdc.cambridge, on Instagram ccdc_cambridge or on YouTube CCDCCambridge.
Understand some of the terms and concepts used with our Frequently Asked Questions page here.
A 3D visualization showing Tungsten in a real crystal structure: