Vanadium
Vanadium:

Small amounts of vanadium make steel markedly stronger. Today, 85% of extracted vanadium is used in steel manufacture.
Facts about Vanadium:
- Vanadium: A ductile, malleable, steel-blue metal
- Fun fact about Vanadium: Elemental vanadium is among the hardest elements known. Only tungsten, chromium, boron and carbon (as a diamond) are harder according to Mohs scale of mineral hardness.
- Chemical symbol: V
- Atomic number: 23
A crystal structure containing Vanadium:
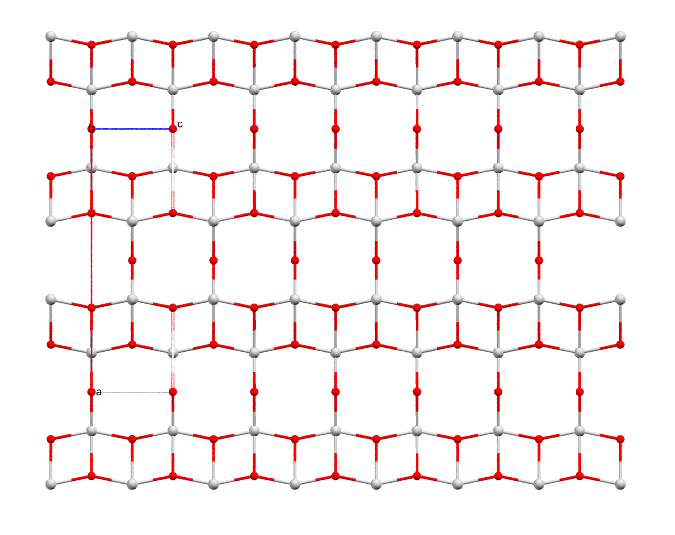
Divanadium pentoxide: the industrial precursor for steel manufacture. Metallic spheres represent vanadium, and red ones oxygen.
Facts about this structure:
- Formula: V2O5
- Structure name: Divanadium pentaoxide
- Fun fact about the structure: Divanadium pentoxide rarely occurs naturally as the mineral shcherbinaite, so the structure of natural shcherbinaite is still unknown. The picture above shows the structure of synthetic shcherbinaite.
- ICSD number: 653926 (Find out more about the ICSD database)
- Associated publication: Anders Byström, Karl-Axel Wilhelmi, Otto Brotzen, Acta Chemica Scandinavica, 1950, 4, 1119, DOI: 10.3891/acta.chem.scand.04-1119
More about Vanadium:
Vanadium was discovered twice. Andrés Manuel del Río discovered it in 1801 and wanted to call it erythronium (“the red element”), but French chemists believed he found an impure chromium ore. Friedrich Wöhler, the father of organic chemistry, was close to discovering vanadium in 1831, but did not fully pursue it. Nils Gabriel Sefström finally (re)discovered it in 1831. He named it after the Norse goddess of beauty – Vanadis, who is today better known as Freya. In modern day vanadium compounds are used in batteries, catalysis and, of course, steel manufacture. Marine organisms like algae and sea squirts also use it, for bio-catalysis. The mushroom fly agaric stores it in a compound called amavadin. Rats need it to grow. We do not know if it is an essential nutrient for humans, but large amounts of its compounds are toxic.
Learn More About the International Year of the Periodic Table (IYPT) in Crystals Project:
This project (#IYPTCrystals) is part of the International Year of the Periodic Table celebration (#IYPT2019), read more about the project here.
You can follow us on social media; search for #IYPTCrystals or follow The CCDC on X @ccdc_cambridge on Facebook ccdc.cambridge, on Instagram ccdc_cambridge or on YouTube CCDCCambridge.
Understand some of the terms and concepts used with our Frequently Asked Questions page here.
A 3D visualization showing Vanadium in a real crystal structure: